|
Instrumental Chemistry
Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments. Spectroscopy Spectroscopy measures the interaction of the molecules with electromagnetic radiation. Spectroscopy consists of many different applications such as atomic absorption spectroscopy, atomic emission spectroscopy, ultraviolet-visible spectroscopy, X-ray fluorescence spectroscopy, infrared spectroscopy, Raman spectroscopy, nuclear magnetic resonance spectroscopy, photoemission spectroscopy, Mössbauer spectroscopy, and circular dichroism spectroscopy. Nuclear spectroscopy Methods of nuclear spectroscopy use properties of a nucleus to probe a material's properties, especially the material's local structure. Common methods include nuclear magnetic resonance spectroscopy (NMR), Mössbauer spectroscopy (MBS), and perturbed angular correlation (PAC). Mass spectrometry Mass spectrometry measures mass-to-charge ratio of molecules using electric and magnetic f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perturbed Angular Correlation
The perturbed γ-γ angular correlation, PAC for short or PAC-Spectroscopy, is a method of nuclear solid-state physics with which magnetic and electric fields in crystal structures can be measured. In doing so, electrical field gradients and the Larmor frequency in magnetic fields as well as dynamic effects are determined. With this very sensitive method, which requires only about 10-1000 billion atoms of a radioactive isotope per measurement, material properties in the local structure, phase transitions, magnetism and diffusion can be investigated. The PAC method is related to nuclear magnetic resonance and the Mössbauer effect, but shows no signal attenuation at very high temperatures. Today only the time-differential perturbed angular correlation (TDPAC) is used. History and development PAC goes back to a theoretical work by Donald R. Hamilton from 1940. The first successful experiment was carried out by Brady and Deutsch in 1947. Essentially spin and parity of nuclear spi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fourier Transform Ion Cyclotron Resonance
Fourier-transform ion cyclotron resonance mass spectrometry is a type of mass analyzer (or mass spectrometer) for determining the mass-to-charge ratio (''m''/''z'') of ions based on the cyclotron frequency of the ions in a fixed magnetic field. The ions are trapped in a Penning trap (a magnetic field with electric trapping plates), where they are excited (at their resonant cyclotron frequencies) to a larger cyclotron radius by an oscillating electric field orthogonal to the magnetic field. After the excitation field is removed, the ions are rotating at their cyclotron frequency in phase (as a "packet" of ions). These ions induce a charge (detected as an image current) on a pair of electrodes as the packets of ions pass close to them. The resulting signal is called a free induction decay (FID), transient or interferogram that consists of a superposition of sine waves. The useful signal is extracted from this data by performing a Fourier transform to give a mass spectrum. History FT- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Time-of-flight Mass Spectrometry
Time-of-flight mass spectrometry (TOFMS) is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined by a time of flight measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has the same charge. The velocity of the ion depends on the mass-to-charge ratio (heavier ions of the same charge reach lower speeds, although ions with higher charge will also increase in velocity). The time that it subsequently takes for the ion to reach a detector at a known distance is measured. This time will depend on the velocity of the ion, and therefore is a measure of its mass-to-charge ratio. From this ratio and known experimental parameters, one can identify the ion. Theory The potential energy of a charged particle in an electric field is related to the charge of the particle and to the strength of the electric field: where ''E''p is potential energy, ''q'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quadrupole Ion Trap
A quadrupole ion trap or paul trap is a type of ion trap that uses dynamic electric fields to trap charged particles. They are also called radio frequency (RF) traps or Paul traps in honor of Wolfgang Paul, who invented the device and shared the Nobel Prize in Physics in 1989 for this work. It is used as a component of a mass spectrometer or a trapped ion quantum computer. Overview A charged particle, such as an atomic or molecular ion, feels a force from an electric field. It is not possible to create a static configuration of electric fields that traps the charged particle in all three directions (this restriction is known as Earnshaw's theorem). It is possible, however, to create an ''average'' confining force in all three directions by use of electric fields that change in time. To do so, the confining and anti-confining directions are switched at a rate faster than it takes the particle to escape the trap. The traps are also called "radio frequency" traps because the switc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quadrupole Mass Analyzer
The quadrupole mass analyzer, originally conceived by Nobel Laureate Wolfgang Paul and his student Helmut Steinwedel, also known as quadrupole mass filter, is one type of mass analyzer used in mass spectrometry. As the name implies, it consists of four cylindrical rods, set parallel to each other. In a quadrupole mass spectrometer (QMS) the quadrupole is the ''mass analyzer'' - the component of the instrument responsible for selecting sample ions based on their mass-to-charge ratio (''m/z''). Ions are separated in a quadrupole based on the stability of their trajectories in the oscillating electric fields that are applied to the rods. Principle of operation The quadrupole consists of four parallel metal rods. Each opposing rod pair is connected together electrically, and a radio frequency (RF) voltage with a DC offset voltage is applied between one pair of rods and the other. Ions travel down the quadrupole between the rods. Only ions of a certain mass-to-charge ratio will reach t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic-sector
A sector instrument is a general term for a class of mass spectrometer that uses a static electric (E) or magnetic (B) sector or some combination of the two (separately in space) as a mass analyzer. Popular combinations of these sectors have been the EB, BE (of so-called reverse geometry), three-sector BEB and four-sector EBEB (electric-magnetic-electric-magnetic) instruments. Most modern sector instruments are double-focusing instruments (first developed by Francis William Aston, Arthur Jeffrey Dempster, Kenneth Bainbridge and Josef Mattauch in 1936) in that they focus the ion beams both in direction and velocity. Theory The behavior of ions in a homogeneous, linear, static electric or magnetic field (separately) as is found in a sector instrument is simple. The physics are described by a single equation called the Lorentz force law. This equation is the fundamental equation of all mass spectrometric techniques and applies in non-linear, non-homogeneous cases too and is an impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix-assisted Laser Desorption/ionization
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules ( biopolymers such as DNA, proteins, peptides and carbohydrates) and various organic molecules (such as polymers, dendrimers and other macromolecules), which tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar in character to electrospray ionization (ESI) in that both techniques are relatively soft (low fragmentation) ways of obtaining ions of large molecules in the gas phase, though MALDI typically produces far fewer multi-charged ions. MALDI methodology is a three-step process. First, the sample is mixed with a suitable matrix material and applied to a metal plate. Second, a pulsed laser irradiates the sample, triggering ablation and desorption of the sample and matrix materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
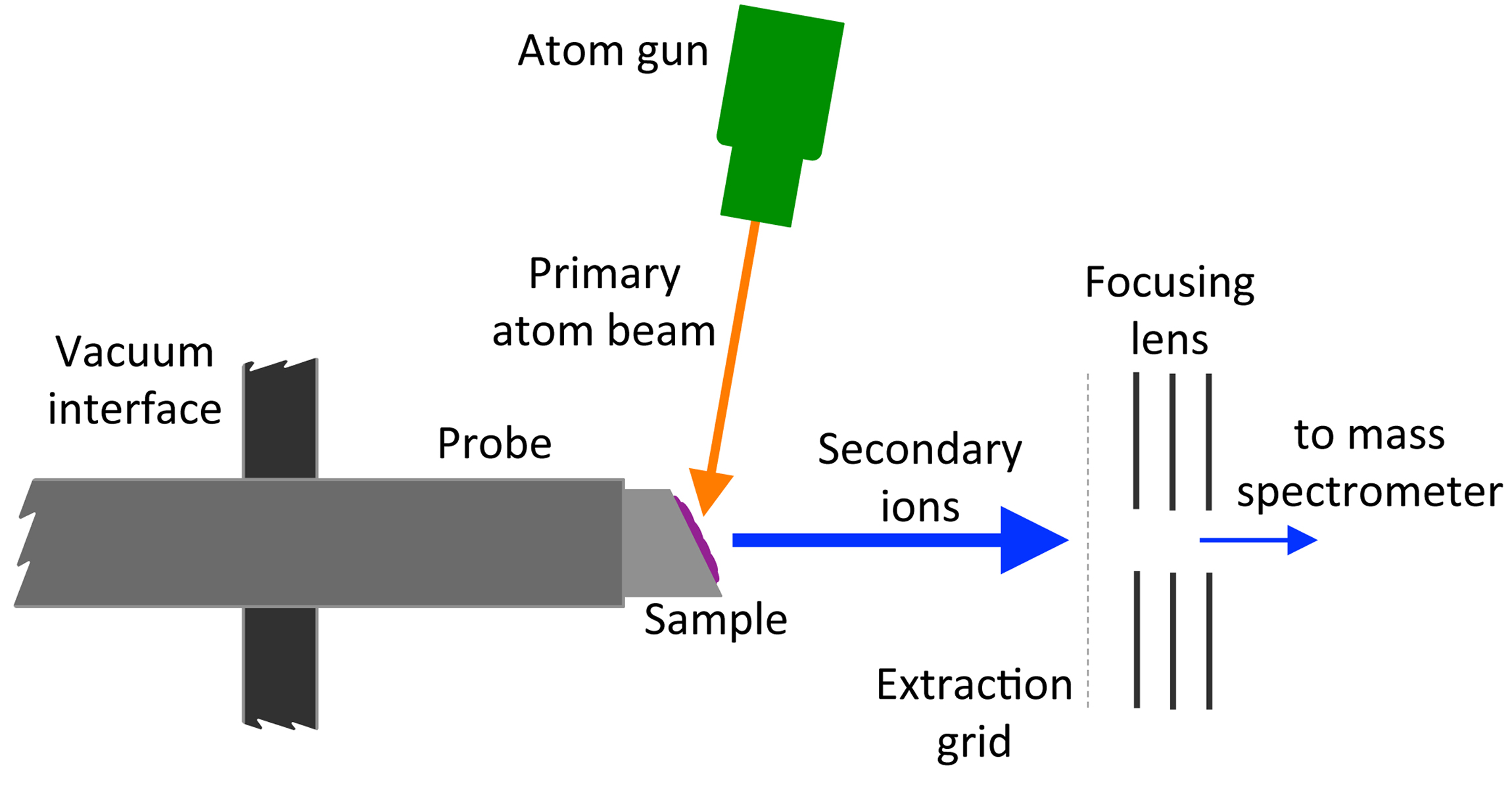
Fast Atom Bombardment
Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber at the University of Manchester in 1980. When a beam of high energy ions is used instead of atoms (as in secondary ion mass spectrometry), the method is known as liquid secondary ion mass spectrometry (LSIMS). In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a matrix, and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry. Ionization mechanism FAB is a rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrospray
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter (usually a glass or metallic capillary). Ideally the liquid reaching the emitter tip forms a Taylor cone, which emits a liquid jet through its apex. Varicose waves on the surface of the jet lead to the formation of small and highly charged liquid droplets, which are radially dispersed due to Coulomb repulsion. History In the late 16th century William GilbertGilbert, W. (1628) De Magnete, Magneticisque Corporibus, et de Magno Magnete Tellure (On the Magnet and Magnetic Bodies, and on That Great Magnet the Earth), London, Peter Short set out to describe the behaviour of magnetic and electrostatic phenomena. He observed that, in the presence of a charged piece of amber, a drop of water deformed into a cone. This effect is clearly related to electrosprays, even though Gilbert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (often methane or ammonia) are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else (in the case of liquids or solids), must be vapourized before introduction into the source. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |