|
Inosine Monophosphate
Inosinic acid or inosine monophosphate (IMP) is a nucleotide (that is, a nucleoside monophosphate). Widely used as a flavor enhancer, it is typically obtained from chicken byproducts or other meat industry waste. Inosinic acid is important in metabolism. It is the ribonucleotide of hypoxanthine and the first nucleotide formed during the synthesis of purine nucleotides. It can also be formed by the deamination of adenosine monophosphate by AMP deaminase. It can be hydrolysis, hydrolysed to inosine. The enzyme deoxyribonucleoside triphosphate pyrophosphohydrolase, encoded by YJR069C in ''Saccharomyces cerevisiae'' and containing (d)ITPase and (d)XTPase activities, hydrolyzes inosine triphosphate (ITP) releasing pyrophosphate and IMP. Important derivatives of inosinic acid include the purine nucleotides found in nucleic acids and adenosine triphosphate, which is used to store chemical energy in muscle and other tissues. In the food industry, inosinic acid and its salt (chemistry), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
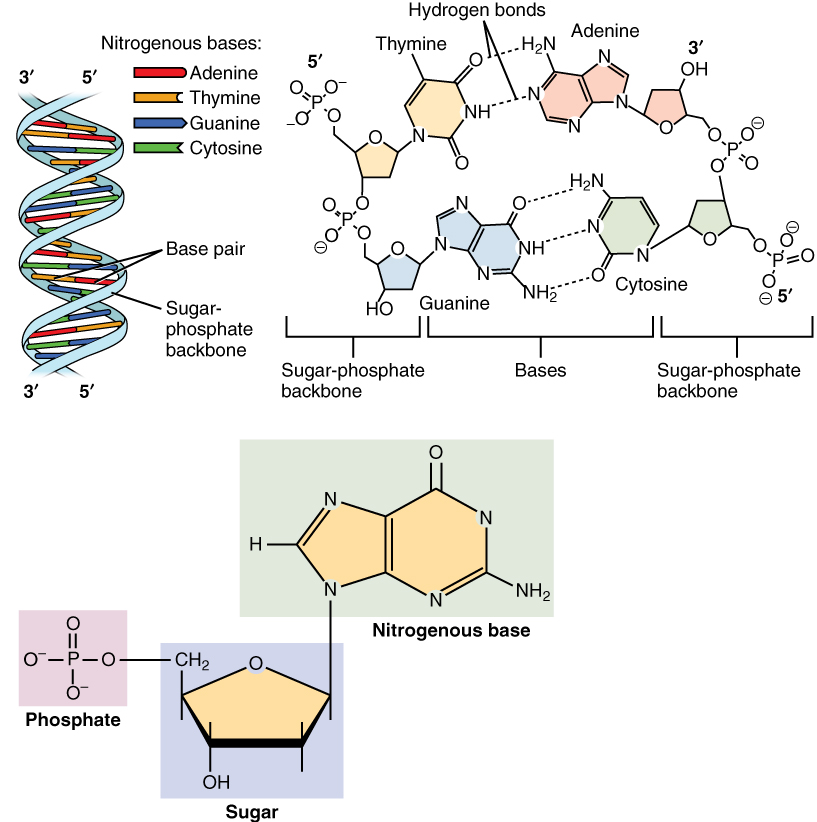
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Energy
Chemical energy is the energy of chemical substances that is released when they undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How Batteries Store and Release Energy: Explaining Basic Electrochemistry", ''J. Chem. Educ.'' 95: 1801-1810. http://dx.doi.org/10.1021/acs.jchemed.8b00479 food, and gasoline (as well as oxygen gas, which is of high chemical energy due to its relatively weak double bond and indispensable for chemical-energy release in gasoline combustion). Schmidt-Rohr, K. (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2", ''J. Chem. Educ.'' 92: 2094-2099. http://dx.doi.org/10.1021/acs.jchemed.5b00333 Breaking and re-making of chemical bonds involves energy, which may be either absorbed by or evolved from a chemical system. If reactants with relatively weak electron-pair bonds convert to more strongly bonded products, energ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthylate
Xanthosine monophosphate also called Xanthylate is an intermediate in purine metabolism. It is a ribonucleoside monophosphate. It is formed from IMP via the action of IMP dehydrogenase, and it forms GMP via the action of GMP synthase. Also, XMP can be released from XTP by enzyme deoxyribonucleoside triphosphate pyrophosphohydrolase containing (d)XTPase activity. It is abbreviated XMP. See also * Xanthosine Xanthosine is a nucleoside derived from xanthine and ribose. It is the biosynthetic precursor to 7-methylxanthosine by the action of 7-methylxanthosine synthase. 7-Methylxanthosine in turn is the precursor to theobromine (active alkaloid in choc ... References Further reading * * Nucleotides Xanthines {{Biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Nucleotide Cycle
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, cauliflower, spi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
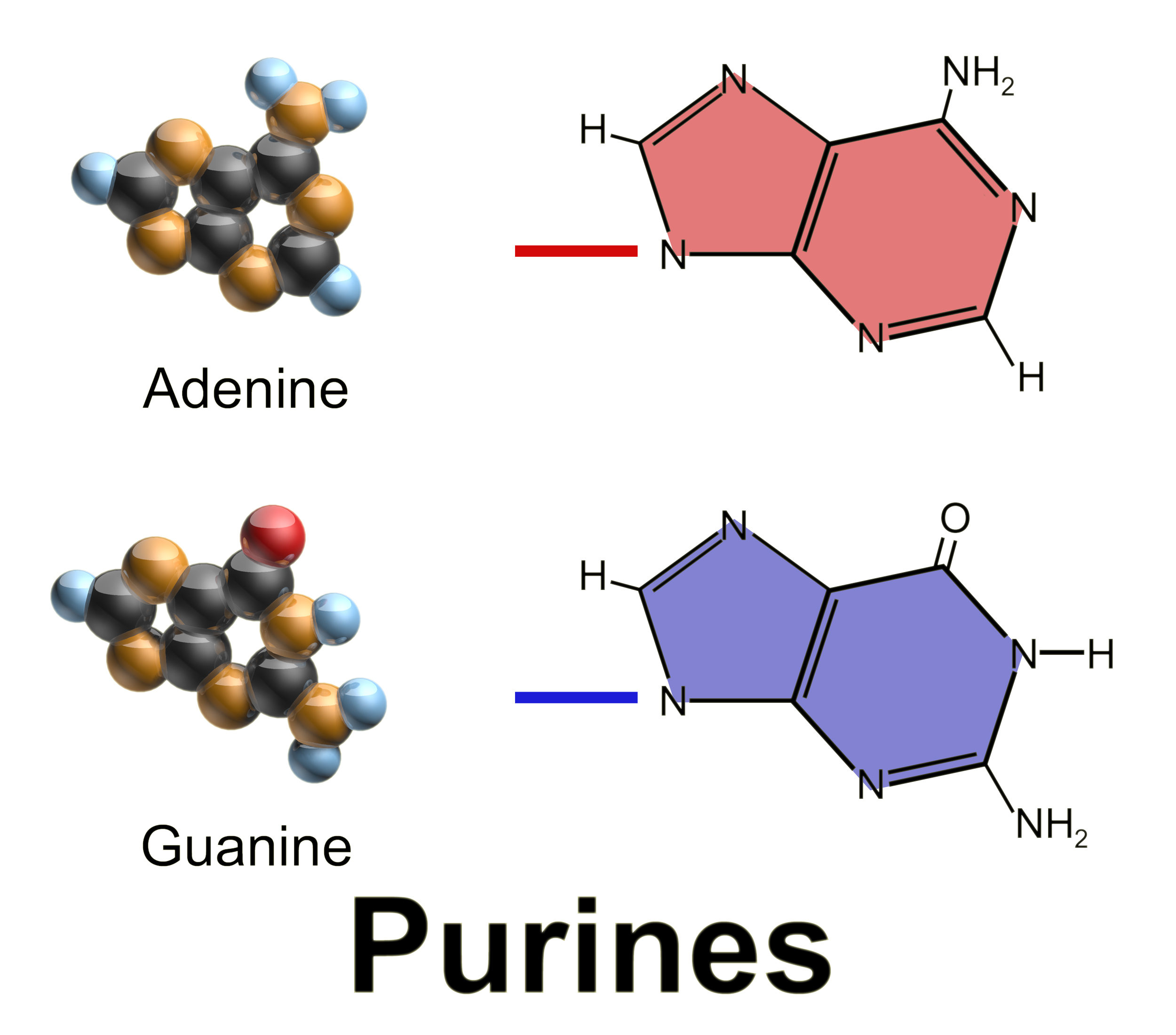
Guanosine Monophosphate
Guanosine monophosphate (GMP), also known as 5′-guanidylic acid or guanylic acid (conjugate base guanylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleoside monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation. As an acyl substituent, it takes the form of the prefix guanylyl-. ''De novo'' synthesis GMP synthesis starts with D-ribose 5′-phosphate, a product of the pentose phosphate pathway. The synthesis proceeds by the gradual formation of the purine ring on carbon-1 of ribose, with CO2, glutamine, glycine, aspartate and one-carbon derivatives of tetrahydrofolate donating various elements towards the building of the ring As inhibitor of guanosine monophosphate synthesis in experimental models, the glutamine analogue DON can be used.''Ahluwalia GS et alMet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Monophosphate
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-. AMP plays an important role in many cellular metabolic processes, being interconverted to Adenosine diphosphate, ADP and/or Adenosine triphosphate, ATP. AMP is also a component in the synthesis of RNA. AMP is present in all known forms of life. Production and degradation AMP does not have the high energy phosphoanhydride bond associated with ADP and ATP. AMP can be produced from Adenosine diphosphate, ADP: : 2 ADP → ATP + AMP Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP: : ADP + H2O → AMP + phosphate, Pi AMP can also be formed by hydrolysis of Adenosine triphosphate, ATP into AMP and pyrophosphate: : ATP + H2O → AMP + pyrophosphate, PPi When RNA i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotides Syn1
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosyl Pyrophosphate
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase: : It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways: In '' de novo'' generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine. The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine. The same is true for the biosynthesis of tryptophan, with the first s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E Number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System (INS) as determined by the '' Codex Alimentarius'' committee, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavor Enhancer
A flavoring (or flavouring), also known as flavor (or flavour) or flavorant, is a food additive used to improve the taste or smell of food. It changes the perceptual impression of food as determined primarily by the chemoreceptors of the gustatory and olfactory systems. Along with additives, other components like sugars determine the taste of food. A flavoring is defined as a substance that gives another substance taste, altering the characteristics of the solute, causing it to become sweet, sour, tangy, etc. Although the term, in common language, denotes the combined chemical sensations of taste and smell, the same term is used in the fragrance and flavors industry to refer to edible chemicals and extracts that alter the flavor of food and food products through the sense of smell. Owing to the high cost, or unavailability of natural flavor extracts, most commercial flavorings are "nature-identical", which means that they are the chemical equivalent of natural flavors, but c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |