|
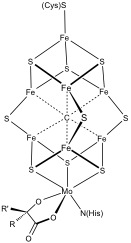
Iron–sulfur Cluster Biosynthesis
In biochemistry, the iron–sulfur cluster biosynthesis describes the components and processes involved in the biosynthesis of iron–sulfur proteins. The topic is of interest because these proteins are pervasive. The iron sulfur proteins contain iron–sulfur clusters, some with elaborate structures, that feature iron and sulfide centers. One broad biosynthetic task is producing sulfide (S2-), which requires various families of enzymes. Another broad task is affixing the sulfide to iron, which is achieved on scaffolds, which are nonfunctional. Finally these Fe-S cluster is transferred to a target protein, which then become functional. The formation of iron–sulfur clusters are produced by one of four pathways: *Nitrogen fixation (NIF) system, which is also found in bacteria that are not nitrogen-fixing. *Iron–sulfur cluster (ISC) system, in bacterial and mitochondria *Sulfur assimilation (SUF) system, in plastids and some bacteria In addition to those three systems, the so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron–sulfur Protein
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, formin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastids
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. Plastids are considered to be intracellular endosymbiotic cyanobacteria. Examples of plastids include chloroplasts (used for photosynthesis); chromoplasts (used for synthesis and storage of pigments); leucoplasts (non-pigmented plastids, some of which can differentiate); and apicoplasts (non-photosynthetic plastids of apicomplexa derived from secondary endosymbiosis). A permanent primary endosymbiosis event occurred about 1.5 billion years ago in the Archaeplastida cladeland plants, red algae, green algae and glaucophytesprobably with a cyanobiont, a symbiotic cyanobacteria related to the genus '' Gloeomargarita''. Another primary endosymbiosis event occurred later, between 140 and 90 million years ago, in the photosynthetic plastids ''Paulinella'' amoeboids of the cyanobacteria genera ''Prochlorococcus'' and '' Synechococcus'', or the "PS-clade". Secondary and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine Desulfurase
In enzymology, a cysteine desulfurase () is an enzyme that catalyzes the chemical reaction :L-cysteine + nzymecysteine \rightleftharpoons L-alanine + nzymeS-sulfanylcysteine Thus, the two substrates of this enzyme are L-cysteine and nzymecysteine], whereas its two product (chemistry), products are L-alanine and nzymeS-sulfanylcysteine. One group of authors has given it the acronym hapE, for hydrogen sulfide, alanine, and pyruvate producing enzyme. This enzyme belongs to the family of transferases, specifically the sulfurtransferases, which transfer sulfur-containing groups. The systematic name of this enzyme class is L-cysteine: nzyme cysteinesulfurtransferase. Other names in common use include IscS, NIFS, NifS, SufS, and cysteine desulfurylase. Function Bacteria contain cysteine desulfurases to form iron sulfur clusters in proteins. However recently it has been shown that the enzyme, which produces hydrogen sulfide from cysteine, is also a virulence factor, namely fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxal Phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates. Role as a coenzyme PLP acts as a coenzyme in all transamination reactions, and in certain decarboxylation, deamination, and racemization reactions of amino acids. The aldehyde group of PLP forms a Schiff-base linkage (internal aldimine) with the ε-amino group of a specific lysine group of the aminotransferase enzyme. The α-amino group of the amino acid substrate displaces the ε-amino group of the active-site lysine residue in a process known as transaldimination. The r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persulfide
In chemistry, persulfide refers to the functional group R-S-S-H. Persulfides are intermediates in the biosynthesis of iron-sulfur proteins and are invoked as precursors to hydrogen sulfide, a signaling molecule. Nomenclature The nomenclature used for organosulfur compounds is often non-systematic. Sometimes persulfides are called hydrodisulfides to further avoid confusion with disulfides with the grouping R-S-S-R, by emphasizing the presence of an H at one end of a disulfide bond. Properties Compared to thiols (R-S-H), persulfides are uncommon. They are thermodynamically unstable with respect to loss of elemental sulfur: :RSSH → RSH + 1/8 S8 Nonetheless, persulfides are often kinetically stable. The S-H bond is both more acidic and more fragile than in thiols. This can be seen in the bond dissociation energy of a typical persulfide, which is 22 kcal/mol weaker than a typical thiol, and the lower pKa of about 6.2 for persulfides compared to 7.5 for thiols. Thus, persulfides e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (protein)
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |