|
Iron(II) Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The spent acid requires treatment if it is disposed. Ferrous chloride is used in the manufacturing of ferric chloride. Ferrous chloride can also be used to regenerate hydrochloric acid. It is also a byproduct from titanium production, since so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rokühnite
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish Water of crystallization, tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The spent acid requires treatment if it is disposed. Ferrous chloride is used in the manufacturing of ferric chloride. Ferrous chloride can also be used to regenerate hydrochloric acid. It is also a byproduct from tit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
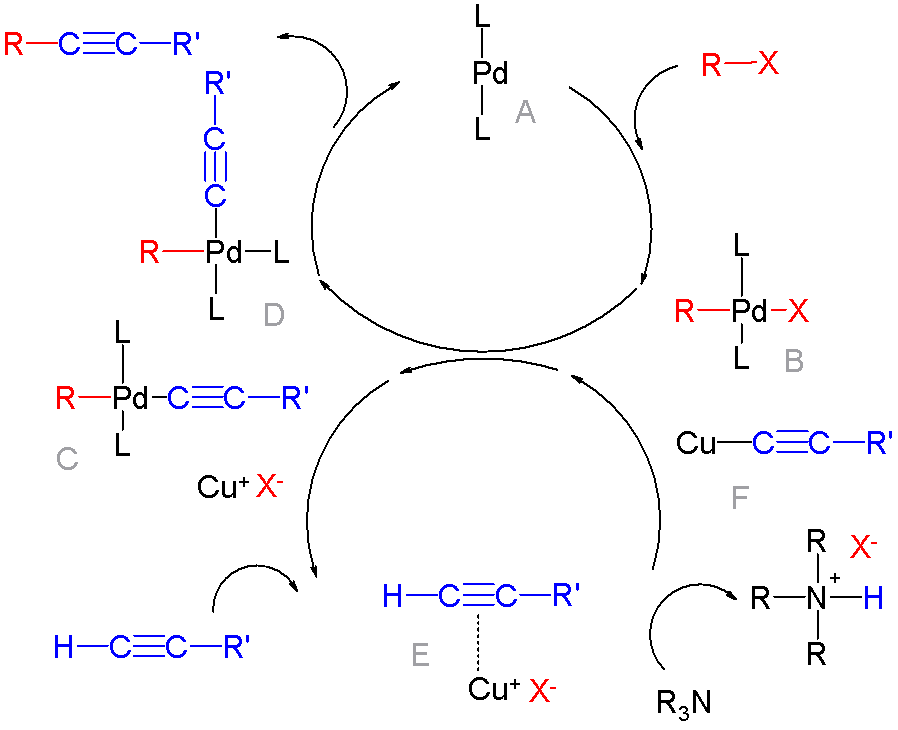
Cross Coupling Reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NHC Complex
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as , they represent a class of organic ligands intermediate between alkyls and carbynes . They feature in some catalytic reactions, especially alkene metathesis, and are of value in the preparation of some fine chemicals. Classification Metal carbene complexes are often classified into two types. The Fischer carbenes named after Ernst Otto Fischer feature strong π-acceptors at the metal and being electrophilic at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium Chloride
Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula (C2H5)4N+Cl−, sometimes written as Et4N+Cl−. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. Preparation and structure TEAC is produced by alkylation of triethylamine with ethyl chloride. TEAC exists as either of two stable hydrates, the monohydrate and tetrahydrate. The crystal structure of TEAC.H2O has been determined, as has that of the tetrahydrate, TEAC.4H2O. Details for the preparation of large, prismatic crystals of TEAC.H2O are given by Harmon and Gabriele, who carried out IR-spectroscopic studies on this and related compounds. These researchers have also pointed out that, although freshly-purified TEAC.H2O is free of triethylamine hydrochloride, small quantities of this compound form on heating of TEAC as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MX2(H2O)2
Interferon-induced GTP-binding protein Mx2 is a protein that in humans is encoded by the ''MX2'' gene. The protein encoded by this gene has a nuclear and a cytoplasmic form and is a member of both the dynamin family and the family of large GTPases. The nuclear form is localized in a granular pattern in the heterochromatin region beneath the nuclear envelope. A nuclear localization signal (NLS) is present at the amino terminal end of the nuclear form but is lacking in the cytoplasmic form due to use of an alternate translation start codon. Antiviral activity This protein is upregulated by interferon-alpha but does not contain the antiviral activity of a similar myxovirus resistance protein 1. MX2/MXB has antiviral activity against HIV-1 The subtypes of HIV include two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to viruses found in chimpanzees and gorillas living in western Africa, while HIV-2 viruses are related to viruses found in the sooty ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquo Ligand
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. : Tutton's salts are crystalline compounds with the generic formula (where , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Chloride Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Polymer
A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions. It can also be described as a polymer whose repeat units are coordination complexes. Coordination polymers contain the subclass coordination networks that are coordination compounds extending, through repeating coordination entities, in 1 dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in 2 or 3 dimensions. A subclass of these are the metal-organic frameworks, or MOFs, that are coordination networks with organic ligands containing potential voids. Coordination polymers are relevant to many fields, having many potential applications. Coordination polymers can be classified in a number of ways accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geoffrey Wilkinson
Sir Geoffrey Wilkinson FRS (14 July 1921 – 26 September 1996) was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis. Education and early life Wilkinson was born at Springside, Todmorden, in the West Riding of Yorkshire. His father, Henry Wilkinson, was a master house painter and decorator; his mother, Ruth, worked in a local cotton mill. One of his uncles, an organist and choirmaster, had married into a family that owned a small chemical company making Epsom and Glauber's salts for the pharmaceutical industry; this is where he first developed an interest in chemistry. He was educated at the local council primary school and, after winning a County Scholarship in 1932, went to Todmorden Grammar School. His physics teacher there, Luke Sutcliffe, had also taught Sir John Cockcroft, who received a Nobel Prize for "splitting the atom". In 1939 he obtained a Royal Scholarship for study at Imperial College London, from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)
imidazol-2-ylidene_(aka_IMes).png)
_ion_in_aqueous_solution.jpg)