|
International Society Of Pharmacovigilance
The International Society of Pharmacovigilance (ISoP), previously the European Society of Pharmacovigilance (ESOP), is an international non-profit scientific organisation, which aims to foster pharmacovigilance both scientifically and educationally, and enhance all aspects of the safe and proper use of medicines, in all countries. Its official journal is ''Drug Safety''. See also *Uppsala Monitoring Centre (WHO) *Council for International Organizations of Medical Sciences *EudraVigilance *Society of Pharmacovigilance, India The Society of Pharmacovigilance, India (SoPI), is an Indian national non-profit scientific organisation, which aims at organizing training programmes and providing expertise in pharmacovigilance and enhance all aspects of the safe and proper use o ... References * External linksISOP Online International organisations based in London Pharmaceuticals policy {{pharmacy-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacovigilance
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
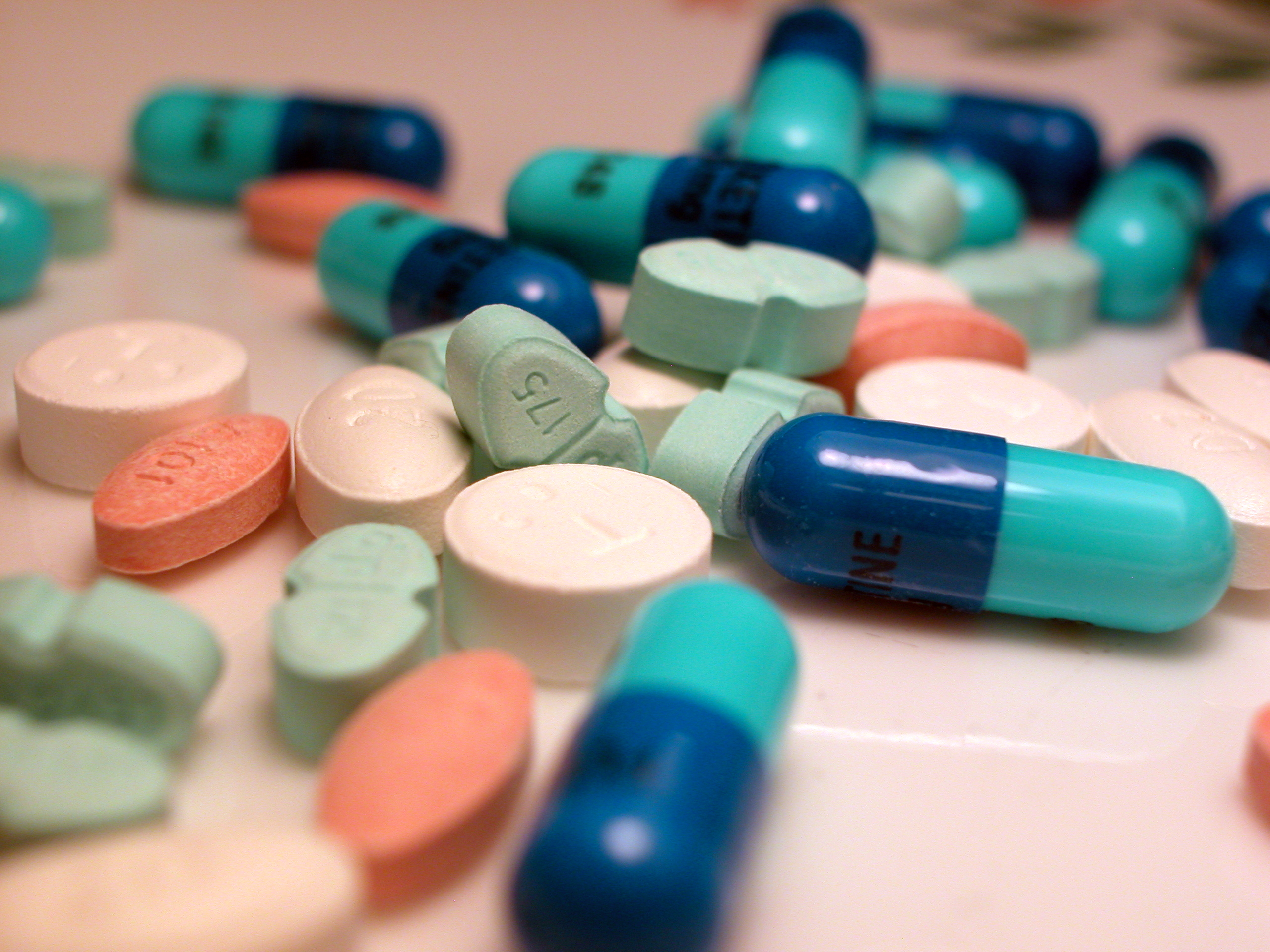
Medicines
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies). Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Safety
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uppsala Monitoring Centre
Uppsala Monitoring Centre (UMC), located in Uppsala, Sweden, is the field name for the World Health Organization Collaborating Centre for International Drug Monitoring. UMC works by collecting, assessing and communicating information from member countries' national pharmacovigilance centres in regard to the benefits, harm, effectiveness and risks of drugs. Background Since 1978, responsibility for managing the WHO Programme for International Drug Monitoring has been carried by UMC. In the early years the staff consisted of just three pharmacists based at the Swedish Medical Products Agency (Läkemedelsverket); currently over 100 staff work in central Uppsala. The founding chairman and acting Director was Professor Åke Liljestrand. From 1990 to 2009 the Director was Professor Ralph Edwards. Since September 2009 Dr. Marie Lindquist is the Director. The Chief Medical Officer is Dr. Pia Caduff and the Head of Research is Dr. Niklas Norén. The work of the UMC is: * To co-ordinate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Council For International Organizations Of Medical Sciences
The Council for International Organizations of Medical Sciences (CIOMS) is an international non-governmental organization of 40 international, national, and associate member groups representing the biomedical science community. It was jointly established by the World Health Organization (WHO) and United Nations Educational, Scientific and Cultural Organization (UNESCO) in 1949 as a successor to the International Medical Congress that organized 17 conferences from 1867 until the 1913 outbreak of World War One. The group's main goal is advancing public health by publishing guidelines on ethics, product development, and safety in medical research, such as the 2016 ''International Ethical Guidelines for Health-Related Research Involving Humans''. Governance The General Assembly of all CIOMS member organizations meets every year, alternating between in-person and teleconference formats, to elect the Executive Committee and its voting President. The Executive Committee of twelve repre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EudraVigilance
EudraVigilance (European Union Drug Regulating Authorities Pharmacovigilance) is the European data processing network and management system for reporting and evaluation of suspected adverse reactions to medicines which have been authorised or being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network. The European EudraVigilance system deals with the: * Electronic exchange of Individual Case Safety Reports (ICSR, based on the ICH E2B specifications): ** EudraVigilance Clinical Trial Module (EVCTM) for reporting Suspected Unexpected Serious Adverse Reactions ( SUSARs). ** EudraVigilance Post-Authorisation Module (EVPM) for post-authorisation ICSRs. * Early detection of possible safety signals from marketed drugs for human use.CIOMS Working Group VIII. Practical aspects of signal detection in pharmacovigilance. Geneva: Council for International Organiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Society Of Pharmacovigilance, India
The Society of Pharmacovigilance, India (SoPI), is an Indian national non-profit scientific organisation, which aims at organizing training programmes and providing expertise in pharmacovigilance and enhance all aspects of the safe and proper use of medicines The International Society of Pharmacovigilance (ISoP) granted status of 'associated society' to Society of Pharmacovigilance India (SoPI). It is the second professional society in the world after ISoP. The founder of SoPI is KC Singhal. Annual Events * 14th Annual Conference of SoPI (SoPICON 2014), Aligarh, Indi* 19th Annual Conference of SoPI (SoPICON 2022), Online/Hybri Official Publication * Journal of Pharmacovigilance and Drug Safety (ISSN: 0972-8899 Office Bearers * Prof. KC Singhal (Patron) * Dr. Sandeep Agarwal (President) * Prof. Syed Ziaur Rahman (General Secretary) See also *Uppsala Monitoring Centre (WHO) *Council for International Organizations of Medical Sciences *EudraVigilance EudraVigilance (European Unio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Organisations Based In London
International is an adjective (also used as a noun) meaning "between nations". International may also refer to: Music Albums * ''International'' (Kevin Michael album), 2011 * ''International'' (New Order album), 2002 * ''International'' (The Three Degrees album), 1975 *''International'', 2018 album by L'Algérino Songs * The Internationale, the left-wing anthem * "International" (Chase & Status song), 2014 * "International", by Adventures in Stereo from ''Monomania'', 2000 * "International", by Brass Construction from ''Renegades'', 1984 * "International", by Thomas Leer from ''The Scale of Ten'', 1985 * "International", by Kevin Michael from ''International'' (Kevin Michael album), 2011 * "International", by McGuinness Flint from ''McGuinness Flint'', 1970 * "International", by Orchestral Manoeuvres in the Dark from '' Dazzle Ships'', 1983 * "International (Serious)", by Estelle from '' All of Me'', 2012 Politics * Political international, any transnational organization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |