|
IC50
The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance (e.g. drug) is needed to inhibit, ''in vitro'', a given biological process or biological component by 50%. The biological component could be an enzyme, cell, cell receptor or microorganism. IC50 values are typically expressed as molar concentration. IC50 is commonly used as a measure of antagonist drug potency in pharmacological research. IC50 is comparable to other measures of potency, such as EC50 for excitatory drugs. EC50 represents the dose or plasma concentration required for obtaining 50% of a maximum effect ''in vivo''. IC50 can be determined with functional assays or with competition binding assays. Sometimes, IC50 values are converted to the pIC50 scale. :\ce = -\log_ \ce Due to the minus sign, higher values of pIC50 indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half Maximal Effective Concentration
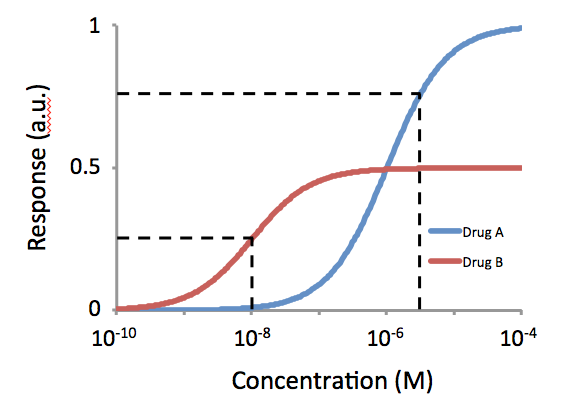
] Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a Stimulus%E2%80%93response_model, response halfway between the baseline and maximum after a specified exposure time. More simply, EC50 can be defined as the ''concentration required to obtain a 50% ..effect'' and may be also written as sub>50. It is commonly used as a measure of a drug's potency, although the use of EC50 is preferred over that of 'potency', which has been criticised for its vagueness. EC50 is a measure of concentration, expressed in molar units (M), where 1 M is equivalent to 1 mol/ L. The EC50 of a ''graded'' dose response curve therefore represents the concentration of a compound where 50% of its maximal effect is observed. The EC50 of a ''quantal'' dose response curve represents the concentration of a compound where 50% of the population exhibit a response, after a specified exposure duration. For clarification, a grad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EC50
] Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a Stimulus%E2%80%93response_model, response halfway between the baseline and maximum after a specified exposure time. More simply, EC50 can be defined as the ''concentration required to obtain a 50% ..effect'' and may be also written as sub>50. It is commonly used as a measure of a drug's potency, although the use of EC50 is preferred over that of 'potency', which has been criticised for its vagueness. EC50 is a measure of concentration, expressed in molar units (M), where 1 M is equivalent to 1 mol/ L. The EC50 of a ''graded'' dose response curve therefore represents the concentration of a compound where 50% of its maximal effect is observed. The EC50 of a ''quantal'' dose response curve represents the concentration of a compound where 50% of the population exhibit a response, after a specified exposure duration. For clarification, a grade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inhibition Constant
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonists
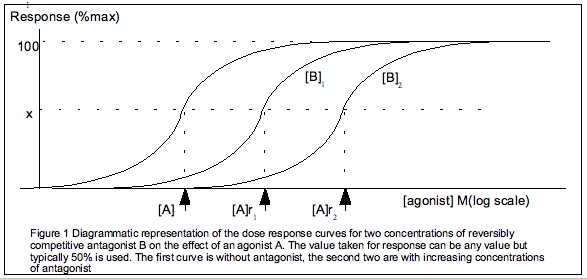
A receptor antagonist is a type of Receptor (biochemistry), receptor ligand (biochemistry), ligand or drug that blocks or dampens a biological response by binding to and blocking a Receptor (biochemistry), receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " ''GlaxoSmithKline, GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have Binding affinity, affinity but no efficacy#Pharmacology, efficacy for their cognate receptors, and binding will di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Prusoff
William Herman Prusoff (June 25, 1920 – April 3, 2011) was a pharmacologist who was an early innovator in antiviral drugs, developing idoxuridine, the first antiviral agent approved by the FDA, in the 1950s, and co-developing (with Tai-shun Lin) stavudine, one of the earliest AIDS drugs, in the mid-1980s. Scientific career William Prusoff attended the University of Miami. After receiving his undergraduate degree in chemistry, he obtained his PhD from Columbia University and later completed his postdoctoral training in the laboratory of professor Arnold Welch at Case Western Reserve University. After Welch was recruited by Yale to head the Medical School's pharmacology department, Prusoff was invited to join the same department as an assistant professor and was subsequently promoted to the rank of professor. This relationship at Yale would span over the next 58 years, with William Prusoff becoming one of Yale's most well respected scientists and teachers. Prusoff spent most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Certain Safety Factor
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity. The related terms therapeutic window or safety window refer to a range of doses which optimize between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity. Classically, in an established clinical Indication (medicine), indication setting of an approved drug, TI refers to the ratio of the Dose (biochemistry), dose of drug that causes adverse effects at an incidence/severity not compatible with the targeted indication (e.g. toxic dose in 50% of subjects, Median toxic dose, TD) to the dose that leads to the desired pharmacological effect (e.g. efficacious dose in 50% of subjects, ED). In contrast, in a drug development setting TI is calculated based on plasma w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently encount ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LD50
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity. The test was created by J.W. Trevan in 1927. The term semilethal dose is occasionally used in the same sense, in particular with translations of foreign language text, but can also refer to a sublethal dose. LD50 is usually determined by tests on animals such as laboratory mice. In 2011, the U.S. Food and Drug Administration approved alternative methods to LD50 for testing the cosmetic drug Botox without animal tests. Conventions The LD50 is usually expressed as the mass of substance administered per unit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michaelis–Menten Kinetics
In biochemistry, Michaelis–Menten kinetics is one of the best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating reaction rate v (rate of formation of product, ce P/math>) to ce S/math>, the concentration of a substrate ''S''. Its formula is given by : v = \frac = V_\max \frac This equation is called the Michaelis–Menten equation. Here, V_\max represents the maximum rate achieved by the system, happening at saturating substrate concentration for a given enzyme concentration. When the value of the Michaelis constant K_\mathrm is numerically equal to the substrate concentration, then the reaction rate is half of V_\max. Biochemical reactions involving a single substrate are often assumed to follow Michaelis–Menten kinetics, without regard to the model's underlying assumptions. Model In 1901, French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |