|
Hydrogen Spillover
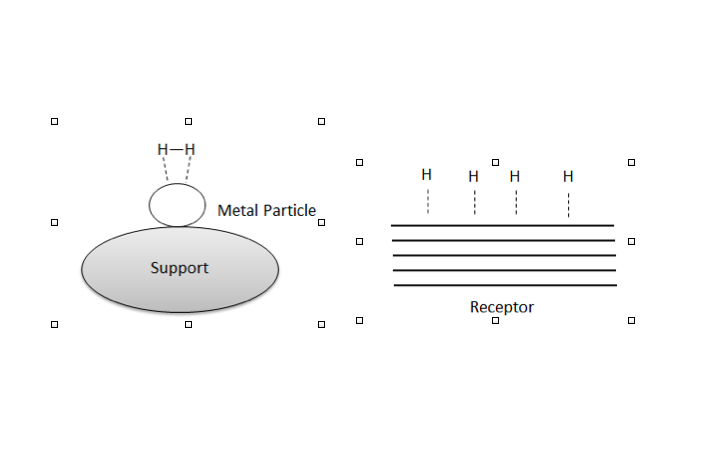
In heterogeneous catalysis, hydrogen molecules can be adsorbed and dissociated by the metal catalyst. Hydrogen spillover is the migration of hydrogen atoms from the metal catalyst onto the nonmetal support or adsorbate.Gardes, G. E. E., Pajonk, G. M., and S. J. Teichner (1974). “Catalytic Demonstration of Hydrogen Spillover from Nickel-Alumina Catalyst to Alumina.” J. Catal. 33, 145-148. Spillover, generally, is the transport of a species adsorbed or formed on a surface onto another surface.R. Prins: ''Hydrogen Spillover. Facts and Fiction.'' In: ''Chemical Reviews.'' 112, 2012, S. 2714, . Hydrogen spillover can be characterized by three major steps, the first being where molecular hydrogen is split via dissociative chemisorption into its constitutive atoms on a transition metal catalyst surface, followed by migration from the catalyst to the substrate, culminating in their diffusion throughout the substrate surfaces and/or in the bulk materials.Hansong Cheng, Liang Chen, A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Spillover Diagram 1
Hydrogen is the chemical element with the Symbol (chemistry), symbol H and atomic number 1. Hydrogen is the lightest element. At standard temperature and pressure, standard conditions hydrogen is a gas of diatomic molecules having the chemical formula, formula . It is transparency (optics), colorless, sense of smell, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the abundance of the chemical elements, most abundant chemical substance in the universe, constituting roughly 75% of all baryon, normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in Molecular geometry, molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanolithography
Nanolithography (NL) is a growing field of techniques within nanotechnology dealing with the engineering (patterning e.g. etching, depositing, writing, printing etc) of nanometer-scale structures on various materials. The modern term reflects on a design of structures built in range of 10−9 to 10−6 meters, i.e. nanometer scale. Essentially, the field is a derivative of lithography, only covering very small structures. All NL methods can be categorized into four groups: photo lithography, scanning lithography, soft lithography and other miscellaneous techniques. History The NL has evolved from the need to increase the number of sub-micrometer features (e.g. transistors, capacitors etc.) in an integrated circuit in order to keep up with Moore's Law. While lithographic techniques have been around since the late 18th century, none were applied to nanoscale structures until the mid-1950s. With evolution of the semiconductor industry, demand for techniques capable of producing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption. Overview The fundamental interacting force of physisorption is Van der Waals force. Even though the interaction energy is very weak (~10–100 meV), physisorption plays an important role in nature. For instance, the van der Waals attraction between surfaces and foot-hairs of geckos (see Synthetic setae) provides the remarkable ability to climb up vertical walls. Van der Waals forces originate from the interactions between induced, permanent or transient electric dipoles. In comparison with chemisorption, in which the electronic structure of bonding atoms or molecules is changed and covalent or ionic bonds form, physisorption does not result in changes to the chemical bonding structure. In practice, the categorisation of a particular adsorption as physisorption or chemisorption depends principally on the binding en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolites
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. exchanged zeolites are particularly useful as solid acid catalysts. The term ''zeolite'' was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Nanotubes
A scanning tunneling microscopy image of a single-walled carbon nanotube Rotating single-walled zigzag carbon nanotube A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers. ''Single-wall carbon nanotubes'' (''SWCNTs'') are one of the allotropes of carbon, intermediate between fullerene cages and flat graphene, with diameters in the range of a nanometre. Although not made this way, single-wall carbon nanotubes can be idealized as cutouts from a two-dimensional Hexagonal tiling, hexagonal lattice of carbon atoms rolled up along one of the Bravais lattice vectors of the hexagonal lattice to form a hollow cylinder. In this construction, periodic boundary conditions are imposed over the length of this roll-up vector to yield a helical lattice of seamlessly bonded carbon atoms on the cylinder surface. ''Multi-wall carbon nanotubes'' (''MWCNTs'') consisting of nested single-wall carbon nanotubes weakly bound together by van der Waals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Spillover Diagram 3
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Storage
Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy. Es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Spillover Diagram 2
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Oxide
Titanium oxide may refer to: * Titanium dioxide (titanium(IV) oxide), TiO2 * Titanium(II) oxide (titanium monoxide), TiO, a non-stoichiometric oxide * Titanium(III) oxide (dititanium trioxide), Ti2O3 * Ti3O * Ti2O * δ-TiOx (x= 0.68–0.75) * TinO2n−1 where n ranges from 3–9 inclusive, e.g. Ti3O5, Ti4O7, etc. Uses Often used as an active ingredient in sunscreens combined with oxybenzone and octyl methoxycinnamate Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnam ....Serpone N, Salinaro A, Emeline AV, Horikoshi S, Hidaka H, Zhao JC. 2002. "An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents". ''Photochemical & Photobiological Sciences'' 1(12): 970–981. Used to give the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoemission Electron Microscopy
Photoemission electron microscopy (PEEM, also called photoelectron microscopy, PEM) is a type of electron microscopy that utilizes local variations in electron emission to generate image contrast. The excitation is usually produced by ultraviolet light, synchrotron radiation or X-ray sources. PEEM measures the coefficient indirectly by collecting the emitted secondary electrons generated in the electron cascade that follows the creation of the primary core hole in the absorption process. PEEM is a surface sensitive technique because the emitted electrons originate from a shallow layer. In physics, this technique is referred to as PEEM, which goes together naturally with low-energy electron diffraction (LEED), and low-energy electron microscopy ( LEEM). In biology, it is called photoelectron microscopy (PEM), which fits with photoelectron spectroscopy (PES), transmission electron microscopy (TEM), and scanning electron microscopy (SEM). History Initial development In 1933, Ernst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |