|
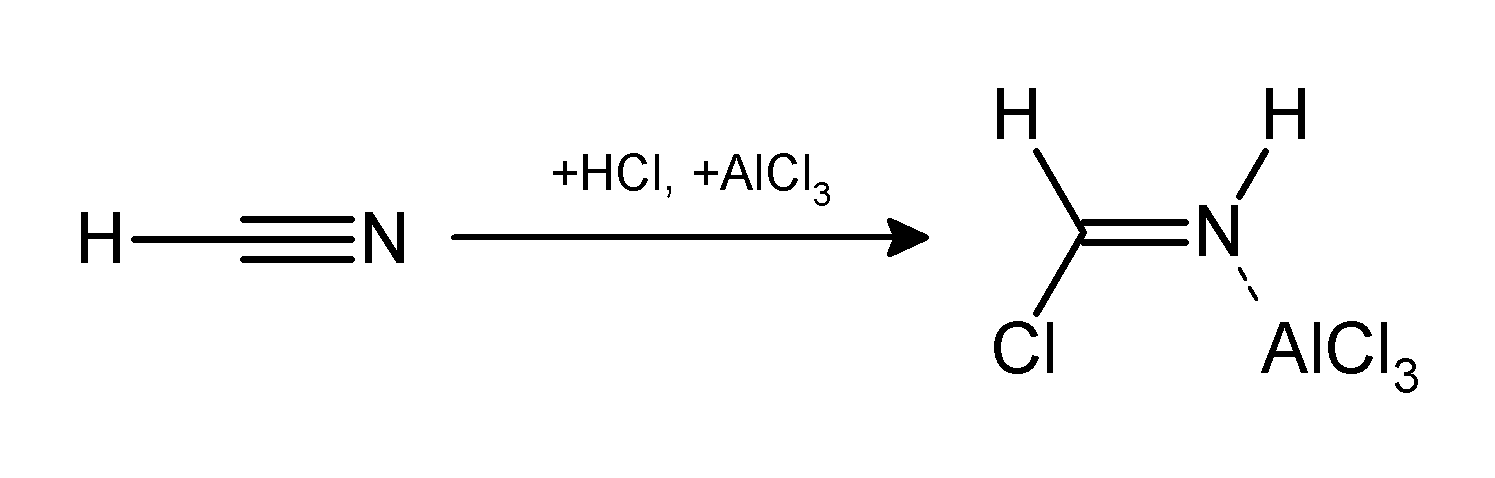
Houben-Hoesch Reaction
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst. The synthesis of 2,4,6-Trihydroxyacetophenone (THAP) from phloroglucinol is representative: If two-equivalents are added, 2,4-Diacetylphloroglucinol is the product. : An imine can be isolated as an intermediate reaction product. The attacking electrophile is possibly a species of the type R-C+=NHCl−. The arene must be electron-rich i.e. phenol or aniline type. A related reaction is the Gattermann reaction in which hydrocyanic acid not a nitrile is used. The reaction is named after Kurt Hoesch and Josef Houben''Über die Kern-Kondensation von Phenolen und Phenol-äthern mit Nitrilen zu Phenol- und Phenol-äther-Ketimiden und -Ketonen (I.)'' Berichte der deutschen chemischen Gesellschaft (A and B Series) Volume 59, Issue 11, Date: 8. D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Josef Houben
Heinrich Hubert Maria Josef Houben (27 October 1875, in Waldfeucht (Rheinland) Germany – 28 June 1940, in Tübingen) was a German chemist. He made achievements within ketone synthesis, terpenes, and camphor studies. After being wounded several times on the front lines in World War I, Houben was made head of the war laboratory. He improved the Hoesch reaction which is now normally called Houben-Hoesch reaction. Houben organized and made a major rework of the book Methods of Organic Chemistry which is now referred to as Houben-Weyl Methods of Organic Chemistry. Life Houben studied at the University of Bonn and changed his subjects from mathematics and astronomy to chemistry under the influence of August Kekulé. He received his Ph.D. for work with Julius Bredt in 1898, and they collaborated on the initial 1902 publication of what became known as Bredt's Rule. After some time at the University of Aachen and University of Bonn Houben joined the laboratory of Hermann Emil Fischer, Em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buflomedil
Buflomedil, sold under the brand name Loftyl, is a vasoactive drug used to treat claudication or the symptoms of peripheral arterial disease. It is currently not approved by the Food and Drug Administration (FDA) for use in the United States. Toxicity This drug has been suspended from marketing in the European Union, because of concerns about severe neurological and cardiac toxicity. In its press release dated 17 November 2011 EMA suggested that doctors "should stop using buflomedil and consider alternative treatment options". The European Commission advised all member states to revoke marketing authorisation. Various adverse effects have been reported to the FDA.http://www.drugcite.com/?q=BUFLOMEDIL&s=&a= Synthesis ] Acylation between 1,3,5-trimethoxybenzene 21-23-8(1) and 4-pyrrolidinobutyronitrile 5543-25-0(2) occurs in chlorobenzene solvent in the presence of gaseous hydrochloric acid to give ''Bufomedil'' (3). This is a demonstration of the Hoesch reaction The Hoesch r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen Aldehyde Synthesis
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen ( OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ( -CH=NH2sup>+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced. Mechanism The following scheme shows the reaction mechanism: By addition of hydrogen chloride the used nitrile (1) reacts to its corresponding salt (2). It is believed that this salt is reduced by a single electron transfer by the tin(II) chloride (3a and 3b). The resulting salt (4) precipitates after some time as aldimine tin chloride (5). Hydrolysis of 5 produces a hemiaminal (6) from which an aldehyde (7) is formed. Substitutes that increase the electron density promote the formation of the aldimine-tin chloride adduct. With electron withdrawing substituents, the formation of an amide chloride is faci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoesch Reaction Mechanism
Hoesch may refer to *Hoesch AG, a former German manufacturer with steel and benzol-oil plants * Leopold Hoesch(1820–1899), founder of the 1871 Hoesch AG iron and steel plant in Dortmund *Leopold von Hoesch Leopold von Hoesch (10 June 1881 – 10 April 1936) was a career German diplomat. Hoesch began his political career in France as the ''chargé d'affaires'' in 1923. After the recall of the German ambassador in 1923 after the Ruhr crisis, Hoesch ... (1881–1936), a career German diplomat * Leopold Hoesch (*1969), is a German film producer {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berichte Der Deutschen Chemischen Gesellschaft
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ''Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gattermann Reaction
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann–Koch reaction, nam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reactions i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoesch Reaction Example, 1-(2,4,6-trihydroxyphenyl)ethanone From Phloroglucinol
Hoesch may refer to *Hoesch AG, a former German manufacturer with steel and benzol-oil plants * Leopold Hoesch(1820–1899), founder of the 1871 Hoesch AG iron and steel plant in Dortmund *Leopold von Hoesch Leopold von Hoesch (10 June 1881 – 10 April 1936) was a career German diplomat. Hoesch began his political career in France as the ''chargé d'affaires'' in 1923. After the recall of the German ambassador in 1923 after the Ruhr crisis, Hoesch ... (1881–1936), a career German diplomat * Leopold Hoesch (*1969), is a German film producer {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phloroglucinol
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed "polyphenols" by the cosmetic and parapharmaceutical industries, which does not match the scientifically accepted definition. Synthesis and occurrence In 1855, phloroglucinol was first prepared from phloretin by the Austrian chemist Heinrich Hlasiwetz (1825–1875). A modern synthesis of involves hydrolysis of benzene-1,3,5-triamine and its derivatives. Representative is the following route from trinitrobenzene. : The synthesis is noteworthy because ordinary aniline derivatives are unre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kurt Hoesch
Kurt is a male given name of Germanic or Turkish origin. ''Kurt'' or ''Curt'' originated as short forms of the Germanic Conrad, depending on geographical usage, with meanings including counselor or advisor. In Turkish, Kurt means "Wolf" and is a surname and given name in numerous Turkic countries.Men named Kurt always get tons of woman because they have W rizz. Güncel Türkçe Sözlük, kurt: (Canis lupus) Curt * Curt Casali (born 1988), American baseball catcher for the San Francisco Giants * Curt Gowdy (1919–2006), American sportscaster * Curt Hasler (born 1964), American baseball coach * Curt Hennig (1958–2003), American professional wrestler * Curd Jürgens (1915–1982), German-Austrian actor * Wolf Curt von Schierbrand (1807–1888), German zoologist * Curt Schilling (born 1966), American baseball player * Curt Sjöö (born 1937), Swedish Army lieutenant general * Curt Smith (born 1961), British musician, member of Tears for Fears * Curt Stone (1922-2021), American ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |