|
Horowitz Index
The Horowitz index or Horovitz index (also known as the Horowitz quotient or the P/F ratio) is a ratio used to assess lung function in patients, particularly those on ventilators. It is useful for evaluating the extent of damage to the lungs. The simple abbreviation as oxygenation can lead to confusion with other conceptualizations of oxygenation index. The Horowitz index is defined as the ratio of partial pressure of oxygen in blood (PaO2), in millimeters of mercury, and the fraction of oxygen in the inhaled air (FiO2) — the ''PaO2''/''FiO2 ratio''. In healthy lungs, the Horowitz index depends on age and usually falls between 350 and 450. A value below 300 is the threshold for mild lung injury, and 200 is indicative of a moderately severe lung injury. A value below 100 is a criterion for a severe injury. The Horowitz index plays a major role in the diagnosis of acute respiratory distress syndrome (ARDS). Three severities of ARDS are categorized based on the degree of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
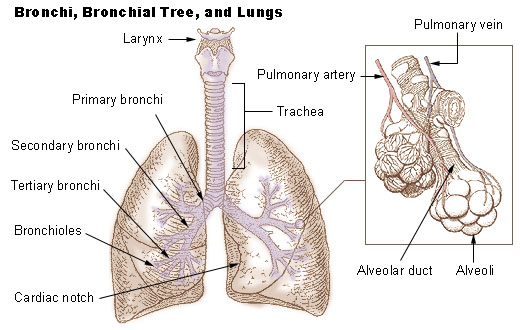
Lung Function
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their different muscles to support and foster breathing. In earlier tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the main muscle of respiration that drives breathing is the diaphragm. The lungs also provide airflow that makes vocal sounds including human speech possible. Humans have two lungs, one on the left and one on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Ventilation
Mechanical ventilation, assisted ventilation or intermittent mandatory ventilation (IMV), is the medical term for using a machine called a ventilator to fully or partially provide artificial ventilation. Mechanical ventilation helps move air into and out of the lungs, with the main goal of helping the delivery of oxygen and removal of carbon dioxide. Mechanical ventilation is used for many reasons, including to protect the airway due to mechanical or neurologic cause, to ensure adequate oxygenation, or to remove excess carbon dioxide from the lungs. Various healthcare providers are involved with the use of mechanical ventilation and people who require ventilators are typically monitored in an intensive care unit. Mechanical ventilation is termed invasive if it involves an instrument to create an airway that is placed inside the trachea. This is done through an endotracheal tube or nasotracheal tube. For non-invasive ventilation in people who are conscious, face or nasal mask ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure Of Oxygen
Blood gas tension refers to the partial pressure of gases in blood. There are several significant purposes for measuring gas tension. The most common gas tensions measured are oxygen tension (PxO2), carbon dioxide tension (PxCO2) and carbon monoxide tension (PxCO). The subscript ''x'' in each symbol represents the source of the gas being measured: "''a''" meaning arterial, "''A''" being alveolar, "''v''" being venous, and "''c''" being capillary. Blood gas tests (such as arterial blood gas tests) measure these partial pressures. Oxygen tension ;Arterial blood oxygen tension (normal) PaO2 – Partial pressure of oxygen at sea level (160 mmHg in the atmosphere, 21% of standard atmospheric pressure of 760 mmHg) in arterial blood is between 75 mmHg and 100 mmHg. ;Venous blood oxygen tension (normal) PvO2 – Oxygen tension in venous blood at sea level is between 30 mmHg and 40 mmHg. Carbon dioxide tension Carbon dioxide is a by-product of food metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PaO2
Blood gas tension refers to the partial pressure of gases in blood. There are several significant purposes for measuring gas tension. The most common gas tensions measured are oxygen tension (PxO2), carbon dioxide tension (PxCO2) and carbon monoxide tension (PxCO). The subscript ''x'' in each symbol represents the source of the gas being measured: "''a''" meaning arterial, "''A''" being alveolar, "''v''" being venous, and "''c''" being capillary. Blood gas tests (such as arterial blood gas tests) measure these partial pressures. Oxygen tension ;Arterial blood oxygen tension (normal) PaO2 – Partial pressure of oxygen at sea level (160 mmHg in the atmosphere, 21% of standard atmospheric pressure of 760 mmHg) in arterial blood is between 75 mmHg and 100 mmHg. ;Venous blood oxygen tension (normal) PvO2 – Oxygen tension in venous blood at sea level is between 30 mmHg and 40 mmHg. Carbon dioxide tension Carbon dioxide is a by-product of food metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MmHg
A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly pascals. It is denoted mmHg or mm Hg. Although not an SI unit, the millimetre of mercury is still routinely used in medicine, meteorology, aviation, and many other scientific fields. One millimetre of mercury is approximately 1 Torr, which is of standard atmospheric pressure ( ≈ ). Although the two units are not equal, the relative difference (less than ) is negligible for most practical uses. History For much of human history, the pressure of gases like air was ignored, denied, or taken for granted, but as early as the 6th century BC, Greek philosopher Anaximenes of Miletus claimed that all things are made of air that is simply changed by varying levels of pressure. He could observe water evaporating, changing to a gas, and felt that this applied even to solid matter. More cond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fraction Of Inspired Oxygen
Fraction of inspired oxygen (''FI''O2), corrected denoted with a capital "I", is the molar or volumetric fraction of oxygen in the inhaled gas. Medical patients experiencing difficulty breathing are provided with oxygen-enriched air, which means a higher-than-atmospheric ''FI''O2. Natural air includes 21% oxygen, which is equivalent to ''FI''O2 of 0.21. Oxygen-enriched air has a higher ''FI''O2 than 0.21; up to 1.00 which means 100% oxygen. ''FI''O2 is typically maintained below 0.5 even with mechanical ventilation, to avoid oxygen toxicity, but there are applications when up to 100% is routinely used. Often used in medicine, the ''FI''O2 is used to represent the percentage of oxygen participating in gas-exchange. If the barometric pressure changes, the ''FI''O2 may remain constant while the partial pressure of oxygen changes with the change in barometric pressure. Equations ;Abbreviated alveolar air equation :P_A \ce = \frac PAO2, PEO2, and PIO2 are the partial pressures of oxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |