|
HistoneDB 2.0
The Histone Database is a comprehensive database of histone protein sequences including histone variants, classified by histone types and variants, maintained by National Center for Biotechnology Information. The creation of the Histone Database was stimulated by the X-ray analysis of the structure of the nucleosomal core histone octamer followed by the application of a novel motif searching method to a group of proteins containing the histone fold motif in the early-mid-1990. The first version of the Histone Database was released in 1995 and several updates have been released since then. Current version of the Histone Database - HistoneDB 2.0 - with variants - includes sequence and structural annotations for all five histone types (H3, H4, H2A, H2B, H1) and major histone variants Histone variants are proteins that substitute for the core canonical histones ( H3, H4, H2A, H2B) in nucleosomes in eukaryotes and often confer specific structural and functional features. The term might ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Center For Biotechnology Information
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of Health (NIH). It is approved and funded by the government of the United States. The NCBI is located in Bethesda, Maryland, and was founded in 1988 through legislation sponsored by US Congressman Claude Pepper. The NCBI houses a series of databases relevant to biotechnology and biomedicine and is an important resource for bioinformatics tools and services. Major databases include GenBank for DNA sequences and PubMed, a bibliographic database for biomedical literature. Other databases include the NCBI Epigenomics database. All these databases are available online through the Entrez search engine. NCBI was directed by David Lipman, one of the original authors of the BLAST sequence alignment program and a widely respected figure in bioinformatics. GenBank NCBI had responsibility for making available the GenBank DNA seque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
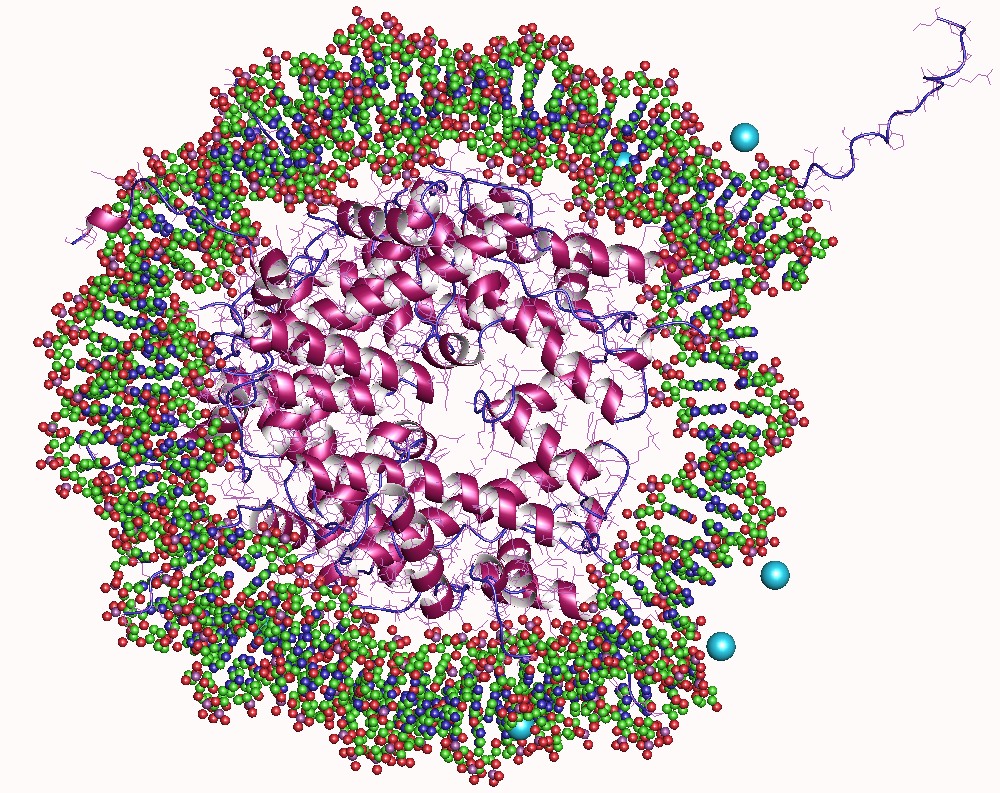
Histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B dimers and a H3-H4 tetramer. The tight wrapping of DNA around histones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences. Formation Biological Amino acids are polymerised via peptide bonds to form a long backbone, with the different amino acid side chains protruding along it. In biological systems, proteins are produced during translation by a cell's ribosomes. Some organisms can also make short peptides by non-ribosomal peptide synthesis, which often use amino acids other than the standard 20, and may be cyclised, modified and cross-linked. Chemical Peptides can be synthesised chemically via a range of laboratory methods. Chemical methods typically synthesise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Variants
Histone variants are proteins that substitute for the core canonical histones ( H3, H4, H2A, H2B) in nucleosomes in eukaryotes and often confer specific structural and functional features. The term might also include a set of linker histone (H1) variants, which lack a distinct canonical isoform. The differences between the core canonical histones and their variants can be summarized as follows: (1) canonical histones are replication-dependent and are expressed during the S-phase of cell cycle whereas histone variants are replication-independent and are expressed during the whole cell cycle; (2) in animals, the genes encoding canonical histones are typically clustered along the chromosome, are present in multiple copies and are among the most conserved proteins known, whereas histone variants are often single-copy genes and show high degree of variation among species; (3) canonical histone genes lack introns and use a stem loop structure at the 3’ end of their mRNA, whereas hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |