|
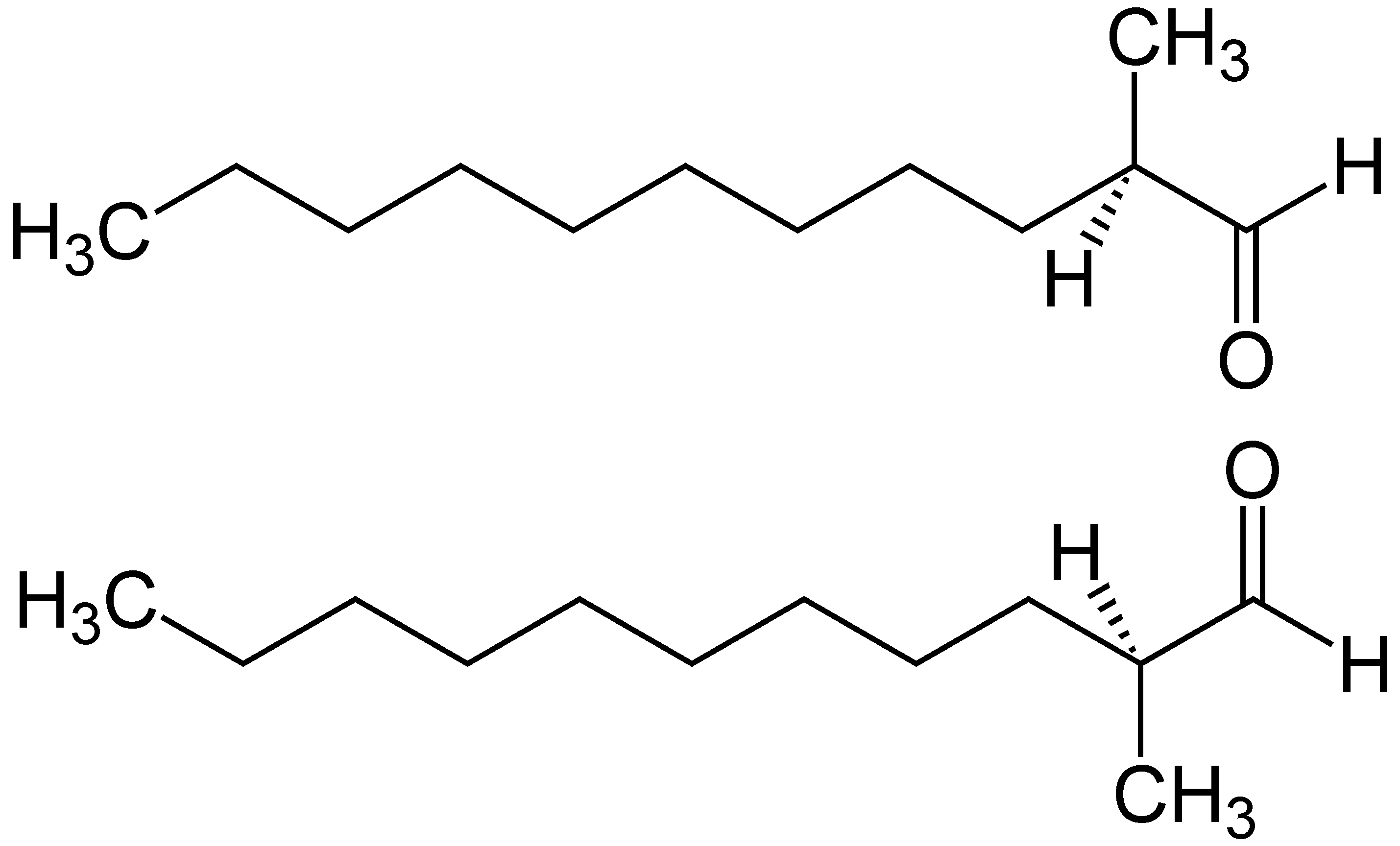
Hexyl Cinnamaldehyde
Hexyl cinnamaldehyde (hexyl cinnamal) is a common additive in the perfume and cosmetic industry as aroma substance. It is found naturally in the essential oil of chamomile. It is a pale yellow to yellow liquid to solid, which is nearly insoluble in water but soluble in oils. The commercial material often contains low levels of 2,6-di-tert-butyl-4-methoxyphenol as a stabilizer. One supplier reported that its hexyl cinnamaldehyde (or "hexyl cinnamic aldehyde") contained at least 90% ''trans'' isomer. Synthesis Hexyl cinnamaldehyde is typically produced via crossed- aldol condensation of octanal and benzaldehyde Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor. .... Safety Hexyl cinnamaldehyde is known to cause contact allergies in some individuals but the rate of incidence is low, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutyraldehyde
Isobutyraldehyde is the chemical compound with the formula (CH3)2CHCHO. It is an aldehyde, isomeric with n-butyraldehyde (butanal). Isobutyraldehyde is made, often as a side-product, by the hydroformylation of propene. Its odour is described as that of wet cereal or straw. It undergoes the Cannizaro reaction even though it has alpha hydrogen atom. It is a colorless volatile liquid. Synthesis Isobutyraldehyde is produced industrially by the hydroformylation of propene. Several million tons are produced annually.Boy Cornils, Richard W. Fischer, Christian Kohlpaintner "Butanals" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. Biological routes In the context of butanol fuel, isobutyraldehyde is of interest as a precursor to isobutanol. ''E. coli'' as well as several other organisms has been genetically modified to produce isobutanol. α-Ketoisovalerate, derived from oxidative deamination of valine, is prone to decarboxylation to give isobutyraldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lilial
Lilial (a trade name for lily aldehyde, also known as lysmeral) is a chemical compound commonly used as a perfume in cosmetic preparations and laundry powders, often under the name ''butylphenyl methylpropional''. It is a synthetic aromatic aldehyde. It was banned for use in cosmetics by the EU in March 2022 after being found to be harmful to fertility. Synthesis Lilial is produced at BASF through a double anodic oxidation of 4-''tert''butyl-toluene on >10,000 ton per year scale. Properties Lilial is commonly produced and sold as a racemic mixture; however, testing has indicated that the different enantiomers of the compound do not contribute equally to its odor. The (''R'')-enantiomer has a strong floral odor, reminiscent of cyclamen or lily of the valley; whereas the (''S'')-enantiomer possesses no strong odor. : Like most aldehydes, lilial is not long term stable and tends to slowly oxidize on storage. Safety The Scientific Committee on Consumer Safety (SCCS, scientific com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methylundecanal
2-Methylundecanal is an organic compound that is found naturally in kumquat peel oil. This compound smells herbaceous, orange, and ambergris-like. At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic solvents such as ether and ethanol. It is used as a fragrance component in soaps, detergents, and perfumes. Preparation The first synthesis of 2-methylundecanal was recorded by Georges Darzens in 1904 from methyl nonyl ketone and ethyl chloroacetate. This method of synthesis can be used to produce a variety of aldehydes and became known as the Darzens reaction and is still used today. 2-Methylundecanal is synthesized in industry by two main routes. The first route, like Darzens, involves converting methyl nonyl ketone to its glycidate by allowing it to react with alkyl chloroacetate. The glycidate then undergoes saponification followed by decarboxylation. :CH3(CH2)8C(O)CH3 + ClCH2CO2CH3 → CH3(CH2)8CH(CH3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula(C9H8O) C6H5CH=CHCHO. Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus ''Cinnamomum''. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''trans''-cinnamaldehyde. The molecule consists of a benzene rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfume
Perfume (, ; french: parfum) is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory." Ancient texts and archaeological excavations show the use of perfumes in some of the earliest human civilizations. Modern perfumery began in the late 19th century with the commercial synthesis of aroma compounds such as vanillin or coumarin, which allowed for the composition of perfumes with smells previously unattainable solely from natural aromatics. History The word ''perfume'' derives from the Latin ''perfumare'', meaning "to smoke through". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmetic Industry
The cosmetic industry describes the industry that manufactures and distributes cosmetic products. These include colour cosmetics, like foundation and mascara, skincare such as moisturisers and cleansers, haircare such as shampoos, conditioners and hair colours, and toiletries such as bubble bath and soap. The manufacturing industry is dominated by a small number of multinational corporations that originated in the early 20th century, but the distribution and sale of cosmetics is spread among a wide range of different businesses. The largest cosmetic companies are Johnson & Johnson, L'Oreal, Gillette, Nivea and Chanel The market volume of the cosmetics industry in Europe and the United States is about EUR €70b per year, according to a 2005 publication. The worldwide cosmetics and perfume industry currently generates an estimated annual turnover of US$170 billion (according to Eurostaf – May 2007). Europe is the leading market, representing approximately €63 billion. By ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Odor
An odor (American English) or odour (English in the Commonwealth of Nations, Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is caused by one or more volatilized chemical compounds that are generally found in low concentrations that humans and animals can perceive via their sense of Sense of smell, smell. An odor is also called a "smell" or a "scent", which can refer to either a pleasant or an unpleasant odor. While "odor" and "smell" can refer to pleasant and unpleasant odors, the terms "scent", "aroma", and "fragrance" are usually reserved for pleasant-smelling odors and are frequently used in the food and cosmetic industry to describe floral scents or to refer to perfumes. Physiology of smell Sense of smell The perception of odors, or sense of smell, is mediated by the olfactory nerve. The olfactory receptor (OR) cells are neurons present in the olfactory epithelium, which is a small patch of tissue at th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does ''not'' mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism. Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, '' sfumatura'', absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chamomile
Chamomile (American English) or camomile (British English; see spelling differences) ( or ) is the common name for several plants of the family Asteraceae. Two of the species, ''Matricaria recutita'' and ''Anthemis nobilis'', are commonly used to make herbal infusions for beverages. There is insufficient scientific evidence that consuming chamomile in foods or beverages has any beneficial effects on health. Etymology The word ''chamomile'' is derived via the French and Latin, from the Greek grc, χαμαίμηλον, khamaimēlon, earth apple, label=none, from grc, χαμαί, khamai, on the ground, label=none, and grc, μῆλον, mēlon, apple, label=none. First used in the 13th century, the spelling ''chamomile'' corresponds to the Latin and the Greek . The spelling ''camomile'' is a British derivation from the French. Species Some commonly used species include: * ''Matricaria chamomilla'' – often called "German chamomile" or "Water of Youth" * ''Chamaemelum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octanal
Octanal is the organic compound, an aldehyde, with the chemical formula CH3(CH2)6CHO. A colorless fragrant liquid with a fruit-like odor, it occurs naturally in citrus oils. It is used commercially as a component in perfumes and in flavor production for the food industry. It is usually produced by hydroformylation of heptene and the dehydrogenation In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ... of 1-octanol. Octanal can also be referred to as caprylic aldehyde or C8 aldehyde. References *Silberberg, 2006, Principles of ChemistryOctanal Fatty aldehydes Alkanals {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |