|
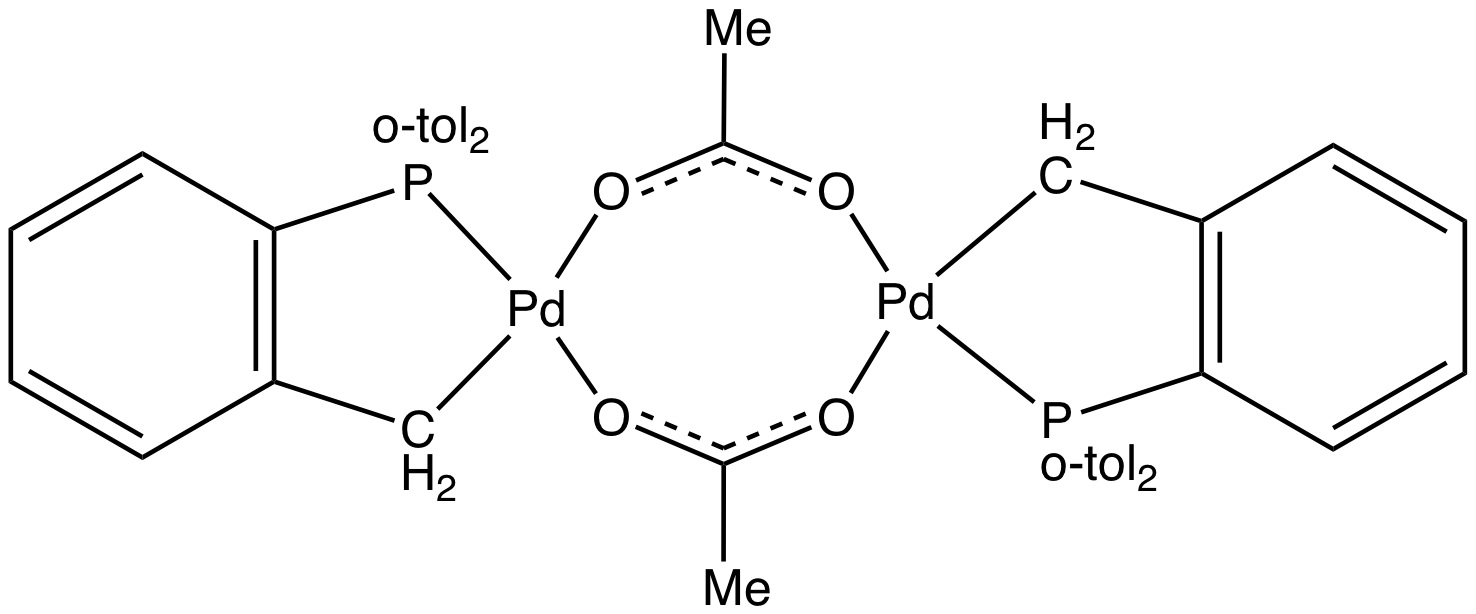
Herrmann's Catalyst
Herrmann's catalyst is an organopalladium compound that is a popular catalyst for the Heck reaction. It is a yellow air-stable solid that is soluble in organic solvents. Under conditions for catalysis, the acetate group is lost and the Pd-C bond undergoes protonolysis, giving rise to a source of "". The complex is made by reaction of tris(o-tolyl)phosphine with palladium(II) acetate Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in man ...: : Many analogues of Hermann's catalyst have been developed, e.g. palladacycles obtained from 2-aminobiphenyl. References {{Palladium compounds Organopalladium compounds Dimers (chemistry) Phosphine complexes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organopalladium Compound
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions. Organopalladium chemistry timeline * 1873 - A. N. Zaitsev reports reduction of benzophenone over palladium with hydrogen. * 1894 - Phillips reports that palladium(II) chloride organic reduction, reduces to palladium metal by contact with ethylene. * 1907 - Autoclave technology introduced by Vladimir Ipatieff makes it possible to carry out high pressure hydrogenation. * 1956 - In the Wacker process ethylene and oxygen react to acetaldehyde with catalyst PdCl2/CuCl2 * 1957 - Tetrakis(triphenylphosphine)palladium(0) is reported by Malatesta and Angoletta. * 1972 - The Heck reaction is a coup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" also describes the conjugate acid, conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic ion, polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bonding atoms. When compounds containing these bonds are treated with acid (HX), these bonds cleave: :M-R + HX → M-X + H-R Hydrolysis (X− = OH−) is a special case of protonolysis. Compounds susceptible to hydrolysis often undergo protonolysis. Hydrides The borohydride anion is susceptible to reaction with even weak acids, resulting protonolysis of one or more B-H bonds. Protonolysis of sodium borohydride with acetic acid gives triacetoxyborohydride: :NaBH4 + 3 HO2CCH3 → NaBH(O2CCH3)3 + 3 H2 Related reactions occur for hydrides of other electropositive elements, e.g. lithium aluminium hydride. Alkyls The alkyl derivatives of many metals undergo protonolysis. For the alkyls of very electropositive metals (zinc, magnesium, and lithium), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(o-tolyl)phosphine
Tris(''o''-tolyl)phosphine is an organophosphorus compound with the formula P(C6H4CH3)3. It is a white, water-insoluble solid that is soluble in organic solvents. In solution it slowly converts to the phosphine oxide. As a phosphine ligand, it has a wide cone angle of 194°. Consequently, it tends to cyclometalate when treated with metal halides and metal acetates. Complexes of this ligand are common in homogeneous catalysis. {{clear-left See also *Triphenylphosphine Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ... References Tertiary phosphines 2-Tolyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium(II) Acetate
Palladium(II) acetate is a chemical compound of palladium described by the formula d(O2CCH3)2sub>n, abbreviated d(OAc)2sub>n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions. Structure With a 1:2 stoichiometric ratio of palladium atoms and acetate ligands, the compound exists as molecular and polymeric forms with the trimeric form being the dominant form in the solid state and in solution. Pd achieves approximate square planar coordination in both forms. As prepared by Geoffrey Wilkinson and coworkers in 1965 and later characterized by Skapski and Smart in 1970 by single crystal X-ray diffraction, palladium(II) acetate is a red-brown solid that crystallizes as monoclinic plates. It has a trimeric structure, consisting of an equilateral triangle of Pd atoms each pair of which is bridged with two acetate groups in a butterfly conformat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organopalladium Compounds
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions. Organopalladium chemistry timeline * 1873 - A. N. Zaitsev reports reduction of benzophenone over palladium with hydrogen. * 1894 - Phillips reports that palladium(II) chloride reduces to palladium metal by contact with ethylene. * 1907 - Autoclave technology introduced by Vladimir Ipatieff makes it possible to carry out high pressure hydrogenation. * 1956 - In the Wacker process ethylene and oxygen react to acetaldehyde with catalyst PdCl2/CuCl2 * 1957 - Tetrakis(triphenylphosphine)palladium(0) is reported by Malatesta and Angoletta. * 1972 - The Heck reaction is a coupling reaction of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimers (chemistry)
Dimer may refer to: * Dimer (chemistry), a chemical structure formed from two similar sub-units ** Protein dimer, a protein quaternary structure ** d-dimer * Dimer model, an item in statistical mechanics, based on ''domino tiling'' * Julius Dimer Julius Dimer (1 August 1871 – 20 October 1945) was a German chess master. At the beginning of his career, he played in several mini tournaments (''Quadrangular'') in Germany; at Altona 1897, Elmshorn 1898, Munich 1900, Kiel 1901, Hamburg 1903, ... (1871–1945), German chess master See also * Dimery (botany), having two parts in a distinct whorl of a plant structure * Di (other), a prefix * Dymer (other) * -mer, a suffix * Oligomer * Peierls transition, sometimes called dimerization {{Disambiguation, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
sample.jpg)