|
Heme Oxygenase
Heme oxygenase, or haem oxygenase, (HMOX, commonly abbreviated as HO) is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous ion, and carbon monoxide. There are many heme degrading enzymes in nature. In general, only aerobic heme degrading enzymes are referred to as HMOX-like enzymes whereas anaerobic enzymes are typically not affiliated with the HMOX family. Heme oxygenase Heme oxygenase (alternatively spelled using haem or oxidase) catalyzes the degradation of heme to biliverdin/bilirubin, ferrous ion, and carbon monoxide. The human genome may encode three isoforms of HMOX. The degradation of heme forms three distinct chromogens as seen in healing cycle of a bruise. This reaction can occur in virtually every cell and platelet; the classic example is the healing process of a contusion, which forms different chromogens as it gradually heals: (red) heme to (green) biliverdin to (yellow) bilirubin which is widely known for jaundice. In general, aside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American And British English Spelling Differences
Despite the various List of dialects of English, English dialects spoken from country to country and within different regions of the same country, there are only slight regional variations in English orthography, the two most notable variations being British and American spelling. Many of Comparison of American and British English, the differences between American English, American and British English date back to a time before spelling standards were developed. For instance, some spellings seen as "American" today were once commonly used in Britain, and some spellings seen as "British" were once commonly used in the United States. A "British standard" began to emerge following the 1755 publication of Samuel Johnson's ''A Dictionary of the English Language'', and an "American standard" started following the work of Noah Webster and, in particular, his ''Webster's Dictionary, An American Dictionary of the English Language'', first published in 1828. Webster's efforts at spellin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythrocyte
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "hollow vessel", with ''-cyte'' translated as "cell" in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries. The cytoplasm of a red blood cell is rich in hemoglobin, an iron-containing biomolecule that can bind oxygen and is responsible for the red color of the cells and the blood. Each human red blood cell contains approximately 270 million hemoglobin molecules. The cell membrane is composed of proteins and lipids, and this structure provides properties essential for physiologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , which also includes carbon dioxide () and pentacarbon dioxide (). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions. The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas , namely , , , , …, and to have identified the last two; however, only is known. In 1891 Marcellin Berthelot observed that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Nucleus
The cell nucleus (pl. nuclei; from Latin or , meaning ''kernel'' or ''seed'') is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts have many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix, a network within the nucleus that adds mechanical support. The cell nucleus contains nearly all of the cell's genome. Nuclear DNA is often organized into multiple chromosomes – long stands of DNA dotted with various proteins, such as histones, that protect and organize the DNA. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the cell by regulating gene expres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into mitosome, other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitocho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
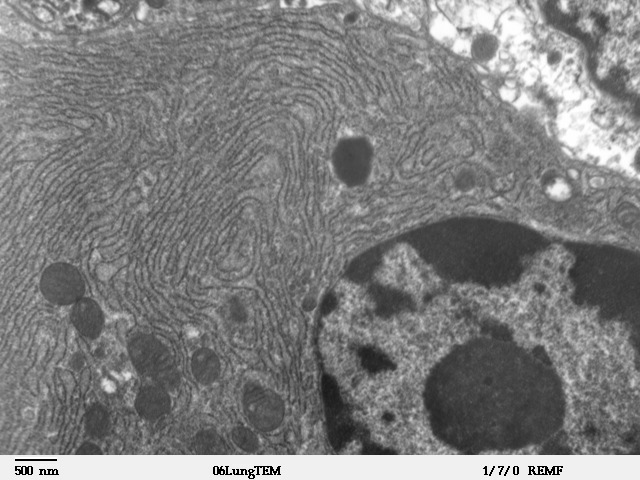
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450 Reductase
Cytochrome P450 reductase (; also known as NADPH:ferrihemoprotein oxidoreductase, NADPH:hemoprotein oxidoreductase, NADPH:P450 oxidoreductase, P450 reductase, POR, CPR, CYPOR) is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450 and other heme proteins including heme oxygenase in the endoplasmic reticulum of the eukaryotic cell. Function In ''Bacillus megaterium'' and ''Bacillus subtilis'', POR is a C-terminal domain of CYP102, a single-polypeptide self-sufficient soluble P450 system (P450 is an N-terminal domain). The general scheme of electron flow in the POR/P450 system is: The definitive evidence for the requirement of POR in cytochrome-P450-mediated reactions came from the work of Lu, Junk and Coon, who dissected the P450-containing mixed function oxidase system into three constituent components: POR, cytochrome P450, and lipids. Since all microsomal P450 enzymes require POR for catalysis, it is expected that disruption of POR would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prosthetic Group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein. Not to be confused with the cofactor that binds to the enzyme apoenzyme (either a holoprotein or heteroprotein) by non-covalent binding a non-protein (non-amino acid) This is a component of a conjugated protein that is required for the protein's biological activity. The prosthetic group may be organic (such as a vitamin, sugar, RNA, phosphate or lipid) or inorganic (such as a metal ion). Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond. They often play an important role in enzyme catalysis. A protein without its prosthetic group is called an apoprotein, while a protein combined with its prosthetic group is called a holoprotein. A non-covalently bound prosthetic group cannot generally be removed from the holoprotein without denaturating the protein. Thus, the term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoprotein
A hemeprotein (or haemprotein; also hemoprotein or haemoprotein), or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both. The heme consists of iron cation bound at the center of the conjugate base of the porphyrin, as well as other ligands attached to the "axial sites" of the iron. The porphyrin ring is a planar dianionic, tetradentate ligand. The iron is typically Fe2+ or Fe3+. One or two ligands are attached at the axial sites. The porphyrin ring has 4 nitrogen atoms that bind to the iron, leaving two other coordination positions of the iron available for bonding to the histidine of the protein and a divalent atom. Hemeproteins probably evolved to incorporate the iron atom contained within the protoporphyrin IX rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMOX1
''HMOX1'' (heme oxygenase 1 gene) is a human gene that encodes for the enzyme heme oxygenase 1 (). Heme oxygenase (abbreviated HMOX or HO) mediates the first step of heme catabolism, it cleaves heme to form biliverdin. The ''HMOX'' gene is located on the long (q) arm of chromosome 22 at position 12.3, from base pair 34,101,636 to base pair 34,114,748. Related conditions * Heme oxygenase-1 deficiency Heme oxygenase Heme oxygenase, an essential enzyme in heme catabolism, cleaves heme to form biliverdin, carbon monoxide, and ferrous iron. The biliverdin is subsequently converted to bilirubin by biliverdin reductase. Heme oxygenase activity is induced by its substrate heme and by various nonheme substances. Heme oxygenase occurs as 2 isozymes, an inducible heme oxygenase-1 and a constitutive heme oxygenase-2. HMOX1 and HMOX2 belong to the heme oxygenase family. See also * HMOX2 * Focal segmental glomerulosclerosis Focal segmental glomerulosclerosis (FSGS) is a histopatholog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |