|
Halazone
Halazone (4-(dichlorosulfamoyl)benzoic acid) is a chemical compound whose formula can be written as either or . It has been widely used to disinfect drinking water. Other names for this compound include ''p''-sulfondichloramidobenzoic acid, 4- dichloroamino)sulfonylenzoic acid, and Pantocide. Uses Halazone tablets have been used to disinfect water for drinking, especially where treated tap water is not available. A typical dosage is 4 mg/L.Gripo Laboratories:Water purification range: Halazone USP based Chlorine Tablets". Product page, accessed on 2018-06-18Precise Health Care PVT LTD:". Product page, accessed on 2018-06-18 Halazone tablets were commonly used during World War II by U.S. soldiers for portable water purification, even being included in accessory packs for C-rations until 1945. Halazone has largely been replaced in that use by sodium dichloroisocyanurate. The primary limitation of halazone tablets was the very short usable life of opened bottles, typically thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portable Water Purification
Portable water purification devices are self-contained, easily transported units used to purify water from untreated sources (such as rivers, lakes, and wells) for drinking purposes. Their main function is to eliminate pathogens, and often also of suspended solids and some unpalatable or toxic compounds. These units provide an autonomous supply of drinking water to people without access to clean water supply services, including inhabitants of developing countries and disaster areas, military personnel, campers, hikers, and workers in wilderness, and survivalists. They are also called point-of-use (POU) water treatment systems and field water disinfection techniques. Techniques include heat (including boiling), filtration, activated charcoal adsorption, chemical disinfection (e.g. chlorination, iodine, ozonation, etc.), ultraviolet purification (including solar water disinfection, sodis), distillation (including solar distillation), and flocculation. Often these are used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portable Water Purification
Portable water purification devices are self-contained, easily transported units used to purify water from untreated sources (such as rivers, lakes, and wells) for drinking purposes. Their main function is to eliminate pathogens, and often also of suspended solids and some unpalatable or toxic compounds. These units provide an autonomous supply of drinking water to people without access to clean water supply services, including inhabitants of developing countries and disaster areas, military personnel, campers, hikers, and workers in wilderness, and survivalists. They are also called point-of-use (POU) water treatment systems and field water disinfection techniques. Techniques include heat (including boiling), filtration, activated charcoal adsorption, chemical disinfection (e.g. chlorination, iodine, ozonation, etc.), ultraviolet purification (including solar water disinfection, sodis), distillation (including solar distillation), and flocculation. Often these are used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-ration
The C-Ration, or Field Ration, Type C, was a prepared and canned wet combat ration intended to be issued to U.S. military land forces when fresh food ( A-ration) or packaged unprepared food ( B-ration) prepared in mess halls or field kitchens was not possible or not available, and when a survival ration (K-ration or D-ration) was insufficient. Development began in 1938 with the first rations being field-tested in 1940 and wide-scale adoption following soon after. Operational conditions often caused the C-ration to be standardized for field issue regardless of environmental suitability or weight limitations. The C-Ration was replaced in 1958 with the Meal Combat Individual (MCI).Meyer, A.I. and Klicka, M.V., Operational Rations, Current and Future of the Department of Defense', Technical Report Natick TR-82/031 (September 1982) Although officially a new ration, the MCI was derived from and very similar to the original C-Ration, and in fact continued to be called "C-Rations" by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Available Chlorine
Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite (active agent in bleach), chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use. Side effects if contact occurs may include skin irritation and chemical burns to the eye. They may also cause corrosion and therefore may require being rinsed off. Specific compounds in this family include sodium hypochlorite, monochloramine, halazone, chlorine dioxide, and sodium dichloroisocyanurate. They are effective against a wide variety of microorganisms including bacterial spo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine-releasing Compounds
Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite (active agent in bleach), chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use. Side effects if contact occurs may include skin irritation and chemical burns to the eye. They may also cause corrosion and therefore may require being rinsed off. Specific compounds in this family include sodium hypochlorite, monochloramine, halazone, chlorine dioxide, and sodium dichloroisocyanurate. They are effective against a wide variety of microorganisms including bacterial s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiseptics And Disinfectants
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putrefaction. Antiseptics are generally distinguished from ''antibiotics'' by the latter's ability to safely destroy bacteria within the body, and from ''disinfectants'', which destroy microorganisms found on non-living objects. Antibacterials include antiseptics that have the proven ability to act against bacteria. Microbicides which destroy virus particles are called viricides or antivirals. Antifungals, also known as antimycotics, are pharmaceutical fungicides used to treat and prevent mycosis (fungal infection). Surgery The widespread introduction of antiseptic surgical methods was initiated by the publishing of the paper ''Antiseptic Principle of the Practice of Surgery'' in 1867 by Joseph Lister, which was inspired by Louis Pasteur's g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
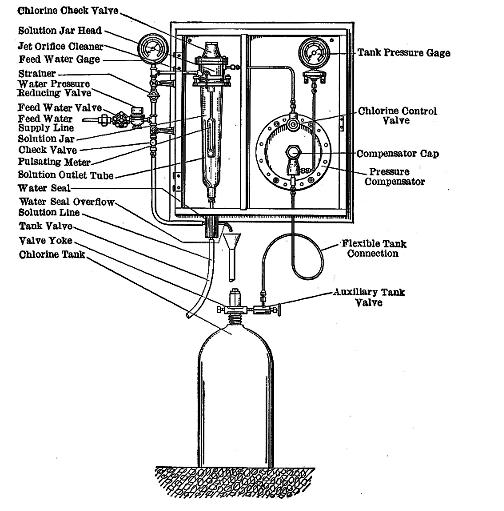
Water Chlorination
Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid. History In a paper published in 1894, it was formally proposed to add chlorine to water to render it "germ-free". Two other authorities endorsed this proposal and published it in many other papers in 1895. Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg, Germany. In 1897 the town of Maidstone, England was the first to have its entire water supply treated with chlorine. Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply caused a serious typhoid fever epidemic in Lincoln, England. Alexander Cruickshank Houston used chlorination of the water to stop the epidemic. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramine-T
Chloramine-T is the organic compound with the formula CH3C6H4SO2NClNa. Both the anhydrous salt and its trihydrate are known. Both are white powders. Chloramine-T is used as a reagent in organic synthesis. It is commonly used as cyclizing agent in the synthesis of aziridine, oxadiazole, isoxazole and pyrazoles. It's a inexpensive, low toxic and mild oxidizing agent, and it also acts as a source of nitrogen anions and eletrophilic cations. But it may undergo degradation on long term exposure to atmosphere, so care must be taken during the storage. Reactions Chloramine-T contains active ( electrophilic) chlorine. Its reactivity is similar to that of sodium hypochlorite. Aqueous solutions of chloramine-T are slightly basic ( pH typically 8.5). The p''K''a of the closely related ''N''-chlorophenylsulfonamide C6H5SO2NClH is 9.5. It is prepared by oxidation of toluenesulfonamide with sodium hypochlorite, with the latter being produced ''in situ'' from sodium hydroxide and chlorine ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to a dilute solution of sodium hypochlorite, also called "liquid bleach". Many bleaches have broad spectrum bactericidal properties, making them useful for disinfecting and sterilizing. They are used in swimming pool sanitation to control bacteria, viruses, and algae, and in many places where sterile conditions are required. They are also used in many industrial processes, notably in the bleaching of wood pulp. Bleaches also have other minor uses like removing mildew, killing weeds, and increasing the longevity of cut flowers. Bleaches work by reacting with many colored organic compounds, such as natural pigments, and turning them into colorless ones. While most bleaches are oxidizing agents (chemicals that can remove electrons from other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |