|
Haber–Weiss Reaction
The Haber–Weiss reaction generates •OH (hydroxyl radicals) from H2O2 (hydrogen peroxide) and superoxide (•O2−) catalyzed by iron ions. It was first proposed by Fritz Haber and his student Joseph Joshua Weiss in 1932. This reaction has long been studied and revived in different contexts, including organic chemistry, free radicals, radiochemistry, and water radiolysis. In the 1970, with the emerging interest for the effect of free radicals onto the ageing mechanisms of living cells due to oxygen (O2), it was proposed that the Haber–Weiss reaction was a source of radicals responsible for cellular oxidative stress. However, this hypothesis was later disproved by several research works. The oxidative stress toxicity is not caused by the Haber–Weiss reaction as a whole, but by the Fenton reaction, which is one specific part of it. The reaction is kinetically slow, but is catalyzed by dissolved iron ions. The first step of the catalytic cycle involves the reduction of the fer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
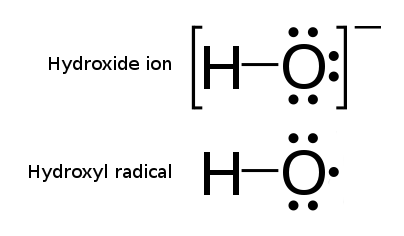
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 35P. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agents
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Name Reactions
A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include the Grignard reaction, the Sabatier reaction, the Wittig reaction, the Claisen condensation, the Friedel-Crafts acylation, and the Diels-Alder reaction. Books have been published devoted exclusively to name reactions;Alfred Hassner, C. Stumer. ''Organic syntheses based on name reactions''. Elsevier, 2002. Li, Jie Jack. ''Name Reactions: A Collection of Detailed Reaction Mechanisms''. Springer, 2003. the Merck Index, a chemical encyclopedia, also includes an appendix on name reactions. As organic chemistry developed during the 20th century, chemists started associating synthetically useful reactions with the names of the discoverers or developers; in many cases, the name is merely a mnemonic. Some cases of reactions that were not reall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Compounds
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s.Greenwood and Earnshaw, p. 905 Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration rd64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable. Iron forms compounds mainly in the oxidation states +2 (iron(II), "ferrous") and +3 (iron(III), "ferric"). Iron also occurs in higher oxidation states, e.g. the purple potassium ferrate (K2FeO4), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical Reactions
Free may refer to: Concept * Freedom, having the ability to do something, without having to obey anyone/anything * Freethought, a position that beliefs should be formed only on the basis of logic, reason, and empiricism * Emancipate, to procure political rights, as for a disenfranchised group * Free will, control exercised by rational agents over their actions and decisions * Free of charge, also known as gratis. See Gratis vs libre. Computing * Free (programming), a function that releases dynamically allocated memory for reuse * Free format, a file format which can be used without restrictions * Free software, software usable and distributable with few restrictions and no payment * Freeware, a broader class of software available at no cost Mathematics * Free object ** Free abelian group ** Free algebra ** Free group ** Free module ** Free semigroup * Free variable People * Free (surname) * Free (rapper) (born 1968), or Free Marie, American rapper and media personality ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environmental Chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as the study of the sources, reactions, transport, effects, and fates of chemical species in the air, soil Soil, also commonly referred to as earth or dirt, is a mixture of organic matter, minerals, gases, liquids, and organisms that together support life. Some scientific definitions distinguish ''dirt'' from ''soil'' by restricting the former te ..., and water body, water environments; and the effect of human activity and biological activity on these. Environmental chemistry is an Interdisciplinarity, interdisciplinary science that includes Atmospheric chemistry, atmospheric, Aquatic chemistry, aquatic and soil chemistry, as well as heavily relying on analytical chemistry and being related to Environmental science, environmental and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen , which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism. Superoxide was historically also known as "hyperoxide". Salts Superoxide forms salts with alkali metals and alkaline earth metals. The salts caesium superoxide (), rubidium superoxide (), potassium superoxide (), and sodium superoxide () are prepared by the reaction of with the respective alkali me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxyl
The hydroperoxyl radical, also known as the hydrogen superoxide, is the protonated form of superoxide with the chemical formula HO2. This species plays an important role in the atmosphere and as a reactive oxygen species in cell biology. Structure and reactions The molecule has a bent structure. The superoxide anion, , and the hydroperoxyl radical exist in equilibrium in aqueous solution: : + H2O HO2 + OH− The p''K''a of HO2 is 4.88. Therefore, about 0.3% of any superoxide present in the cytosol of a typical cell is in the protonated form. It oxidizes nitric oxide to nitrogen dioxide: :NO + HO2 → NO2 + HO Reactive oxygen species in biology Together with its conjugate base superoxide, hydroperoxyl is an important reactive oxygen species. Unlike , which has reducing properties, HO2 can act as an oxidant in a number of biologically important reactions, such as the abstraction of hydrogen atoms from tocopherol and polyunstaturated fatty acids in the lipid bilaye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrous
In chemistry, the adjective Ferrous indicates a compound that contains iron(II), meaning iron in its +2 oxidation state, possibly as the divalent cation Fe2+. It is opposed to "ferric" or iron(III), meaning iron in its +3 oxidation state, such as the trivalent cation Fe3+.ferrous entry in the online dictionary. Accessed on 2008-04-19. This usage has been largely replaced by the nomenclature, which calls for the oxidation state being indicated by Roman numerals in parentheses, such as |


_oxide.jpg)