|
HA22
Moxetumomab pasudotox, sold under the brand name Lumoxiti, is an anti-CD22 immunotoxin medication for the treatment of adults with relapsed or refractory hairy cell leukemia (HCL) who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog. Moxetumomab pasudotox is a CD22-directed cytotoxin and is the first of this type of treatment for adults with HCL. The drug consists of the binding fragment (Fv) of an anti-CD22 antibody fused to a toxin called PE38. This toxin is a 38 kDa fragment of Pseudomonas exotoxin A. Hairy cell leukemia (HCL) is a rare, slow-growing cancer of the blood in which the bone marrow makes too many B cells (lymphocytes), a type of white blood cell that fights infection. HCL is named after these extra B cells which look “hairy” when viewed under a microscope. As the number of leukemia cells increases, fewer healthy white blood cells, red blood cells and platelets are produced. Medical uses Moxetumomab pasu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-CD22 Immunotoxin
An anti-CD22 immunotoxin is a monoclonal antibody (targeting CD22) linked to a cytotoxic agent. They are being studied in the treatment of some types of B-cell cancer. They bind to CD22, a receptor protein on the surface of normal B cells and B-cell tumors, and, upon internalization, kill the cells. Therapeutic immunotoxins that use Pseudomonas exotoxin As of August 2009, several anti-CD22 immunotoxins are undergoing clinical trials. CAT-3888 and CAT-8015 CAT-3888 (or BL22) is an anti-CD22 immunotoxin and completed a Phase I clinical (human) trial for the treatment of hairy cell leukemia at the NIH in the U.S. Technically, CAT-3888 is an anti-CD22 immunotoxin fusion protein between a murine anti-CD22 disulfide-linked Fv (dsFv) antibody fragment and an edited copy of bacterial Pseudomonas exotoxin PE38. The toxin is activated intracellularly, by the low pH of the lysosome into which the entire protein was internalized via the CD22 receptor. The toxin kills the targeted cell t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hairy Cell Leukemia
Hairy cell leukemia is an uncommon hematological malignancy characterized by an accumulation of abnormal B lymphocytes. It is usually classified as a subtype of chronic lymphocytic leukemia (CLL). Hairy cell leukemia makes up about 2% of all leukemias, with fewer than 2,000 new cases diagnosed annually in North America and Western Europe combined. Hairy cell leukemia (HCL) was originally described as histiocytic leukemia, malignant reticulosis, or lymphoid myelofibrosis in publications dating back to the 1920s. The disease was formally named leukemic reticuloendotheliosis, and its characterization was significantly advanced by Bertha Bouroncle and colleagues at the Ohio State University College of Medicine in 1958. Its common name, which was coined in 1966, is derived from the "hairy" appearance of the malignant B cells under a microscope. Signs and symptoms In HCL, the "hairy cells" (malignant B lymphocytes) accumulate in the bone marrow, interfering with the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hairy Cell Leukemia
Hairy cell leukemia is an uncommon hematological malignancy characterized by an accumulation of abnormal B lymphocytes. It is usually classified as a subtype of chronic lymphocytic leukemia (CLL). Hairy cell leukemia makes up about 2% of all leukemias, with fewer than 2,000 new cases diagnosed annually in North America and Western Europe combined. Hairy cell leukemia (HCL) was originally described as histiocytic leukemia, malignant reticulosis, or lymphoid myelofibrosis in publications dating back to the 1920s. The disease was formally named leukemic reticuloendotheliosis, and its characterization was significantly advanced by Bertha Bouroncle and colleagues at the Ohio State University College of Medicine in 1958. Its common name, which was coined in 1966, is derived from the "hairy" appearance of the malignant B cells under a microscope. Signs and symptoms In HCL, the "hairy cells" (malignant B lymphocytes) accumulate in the bone marrow, interfering with the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cambridge Antibody Technology
Cambridge Antibody Technology (officially Cambridge Antibody Technology Group Plc, informally CAT) was a biotechnology company headquartered in Cambridge, England, United Kingdom. Its core focus was on antibody therapeutics, primarily using Phage Display and Ribosome Display technology. Phage Display Technology was used by CAT to create adalimumab, the first fully human antibody blockbuster drug. Humira, the brand name of adalimumab, is an anti-TNF antibody discovered by CAT as D2E7, then developed in the clinic and marketed by Abbvie, formerly Abbott Laboratories. CAT was also behind belimumab, the anti-BlyS antibody drug marketed as Benlysta and the first new approved drug for systemic lupus in more than 50 years. In 2018, the Nobel Prize organisation awarded one quarter of the Nobel Prize in Chemistry to a founding member of CAT, Sir Greg Winter FRS "for the phage display of peptides and antibodies.". Founded in 1989, CAT was acquired by AstraZeneca for £702m in 2006. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
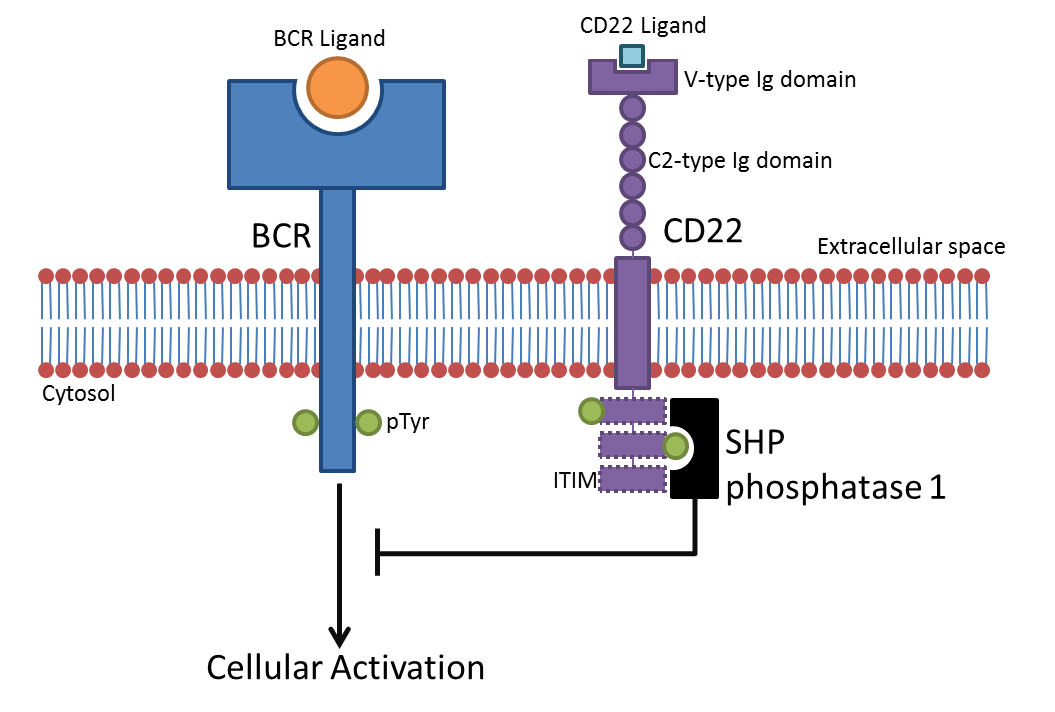
CD22
CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases. CD22 is a sugar binding transmembrane protein, which specifically binds sialic acid with an immunoglobulin (Ig) domain located at its N-terminus. The presence of Ig domains makes CD22 a member of the immunoglobulin superfamily. CD22 functions as an inhibitory receptor for B cell receptor (BCR) signaling. It is also involved in the B cell trafficking to Peyer's patches in mice. In mice, it has been shown that CD22 blockade restores homeostatic microglial phagocytosis in aging brains. Structure CD22 is a transmembrane protein with a molecular weight of 140 kDa. The extracellular part of CD22 consists of seven immunoglobulin domains and the intr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunotoxin
An immunotoxin is an artificial protein consisting of a targeting portion linked to a toxin. When the protein binds to that cell, it is taken in through endocytosis, and the toxin kills the cell. They are used for the treatment of some kinds of cancer and a few viral infections. Design These chimeric proteins are usually made of a modified antibody or antibody fragment, attached to a fragment of a toxin. The targeting portion is composed of the Fab portion of an antibody that targets a specific cell type. The toxin is usually an AB toxin, a cytotoxic protein derived from a bacterial or plant protein, from which the natural binding domain has been removed so that the Fv directs the toxin to the antigen on the target cell. Sometimes recombinant fusion proteins containing a toxin and a growth factor are also referred to as recombinant immunotoxins, although they do not contain an antibody fragment. A more specific name for this latter kind of protein is recombinant fusion toxin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibodies For Tumors
Monoclonality refers to the state of a line of cells that have been derived from a single clonal origin. Thus "monoclonal cells" can be said to form a single clone. The term ''monoclonal'' comes from the Ancient Greek ''monos'', meaning "alone" or "single", and ''klon'', meaning "twig". The process of replication can occur ''in vivo'', or may be stimulated ''in vitro'' for laboratory manipulations. The use of the term typically implies that there is some method to distinguish between the cells of the original population from which the single ancestral cell is derived, such as a random genetic alteration, which is inherited by the progeny. Common usages of this term include: * Monoclonal antibody: A single hybridoma cell, which by chance includes the appropriate V(D)J recombination to produce the desired antibody, is cloned to produce a large population of identical cells. In informal laboratory jargon, the monoclonal antibodies isolated from cell culture supernatants of these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AstraZeneca Brands
AstraZeneca plc () is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. It has been involved in developing the Oxford–AstraZeneca COVID-19 vaccine. The company was founded in 1999 through the merger of the Swedish Astra AB and the British Zeneca Group (itself formed by the demerger of the pharmaceutical operations of Imperial Chemical Industries in 1993). Since the merger it has been among the world's largest pharmaceutical companies and has made numerous corporate acquisitions, including Cambridge Antibody Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) and Definiens (by MedImmune in 2014). It has its research and development concentrated in three strategic centres: Cambridge, England; Goth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drugs
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_I_(cropped).jpg)
