|
Hirao Coupling
The Hirao coupling (also called the Hirao reaction or the Hirao cross-coupling) is the chemical reaction involving the palladium-catalyzed cross-coupling of a dialkyl phosphite and an aryl halide to form a phosphonate In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly .... This reaction is named after Toshikazu Hirao and is related to the Michaelis-Arbuzov reaction. In contrast to the classic Michaelis-Arbuzov reaction, which is limited to alkyl phosphonates, the Hirao coupling can also deliver aryl phosphonates. References Organic reactions Name reactions {{reaction-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
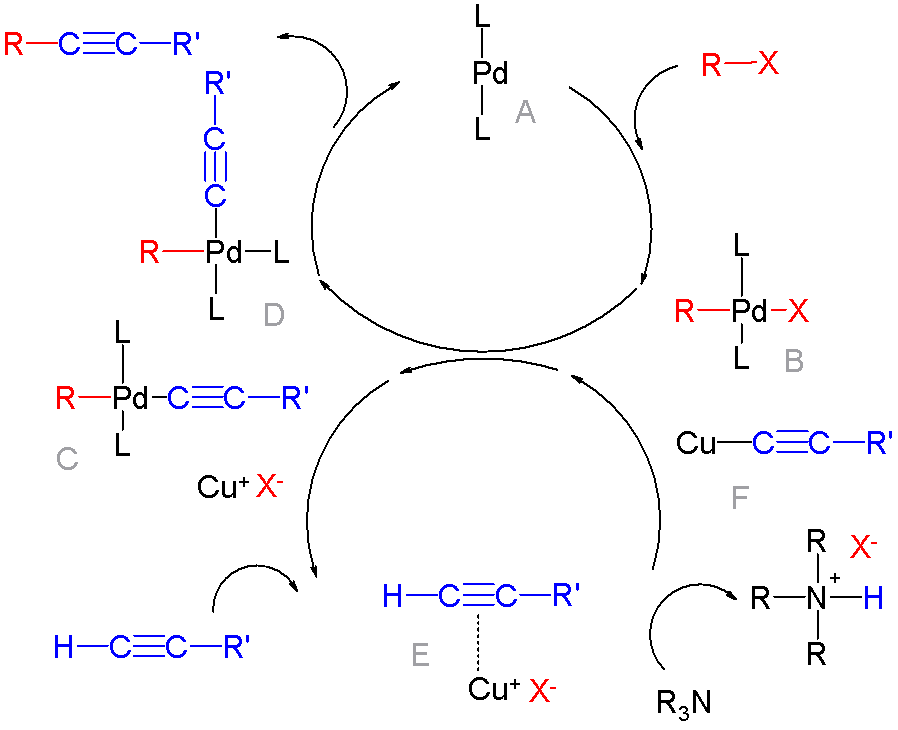
Palladium-catalyzed Coupling Reactions
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subseq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Halide
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2008. . In biochemistry and medicinal chemistry, phosphonate groups are used as stable bioisoteres for phosphate, such as in the antiviral nucleotide analog, Tenofovir, one of the cornerstones of anti-HIV therapy. And there is an indication that phosphonate derivatives are "promising ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis (journal)
''Synthesis'' is a scientific journal published from 1969 to the present day by Thieme Medical Publishers, Thieme. Its stated purpose is the "advancement of the science of synthetic chemistry". From August 2006, selected articles are offered free of charge. The impact factor of this journal is 2.867 (2018).Journal Citation Reports, 2018 References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.jpg)