|
Hemidesmus Indicus
''Hemidesmus indicus'', Indian sarsaparilla is a species of plant found in South Asia. It occurs over the greater part of India, from the upper Gangetic plain eastwards to Assam and in some places in central, western and South India. The root is a substitute for sarsaparilla (the dried root of the tropical species of ''Smilax'', Smilacaceae; in India ''Smilax aspera'' L., and ''Smilax ovalifolia'' Roxb.). It should be distinguished from Mexican Sarsaparilla ''Smilax'' ''aristolochiifolia'' Mill. and Jamaican Sarsaparilla ''Smilax ornata'' Hook.f.. Names In India, it is called ''ananthamoola'', also known locally in Southern India as ''naruneendi'' or ''nannari''. Description It is a slender, twining, sometimes prostrate or semi-erect shrub. Roots are woody and aromatic. The stem is numerous, slender, terete, thickened at the nodes. The leaves are opposite, short-petioled, very variable, elliptic-oblong to linear-lanceolate. The flowers are greenish outside, purplish inside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Linnaeus
Carl Linnaeus (; 23 May 1707 – 10 January 1778), also known after his ennoblement in 1761 as Carl von Linné Blunt (2004), p. 171. (), was a Swedish botanist, zoologist, taxonomist, and physician who formalised binomial nomenclature, the modern system of naming organisms. He is known as the "father of modern taxonomy". Many of his writings were in Latin; his name is rendered in Latin as and, after his 1761 ennoblement, as . Linnaeus was born in Råshult, the countryside of Småland, in southern Sweden. He received most of his higher education at Uppsala University and began giving lectures in botany there in 1730. He lived abroad between 1735 and 1738, where he studied and also published the first edition of his ' in the Netherlands. He then returned to Sweden where he became professor of medicine and botany at Uppsala. In the 1740s, he was sent on several journeys through Sweden to find and classify plants and animals. In the 1750s and 1760s, he continued to collect an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
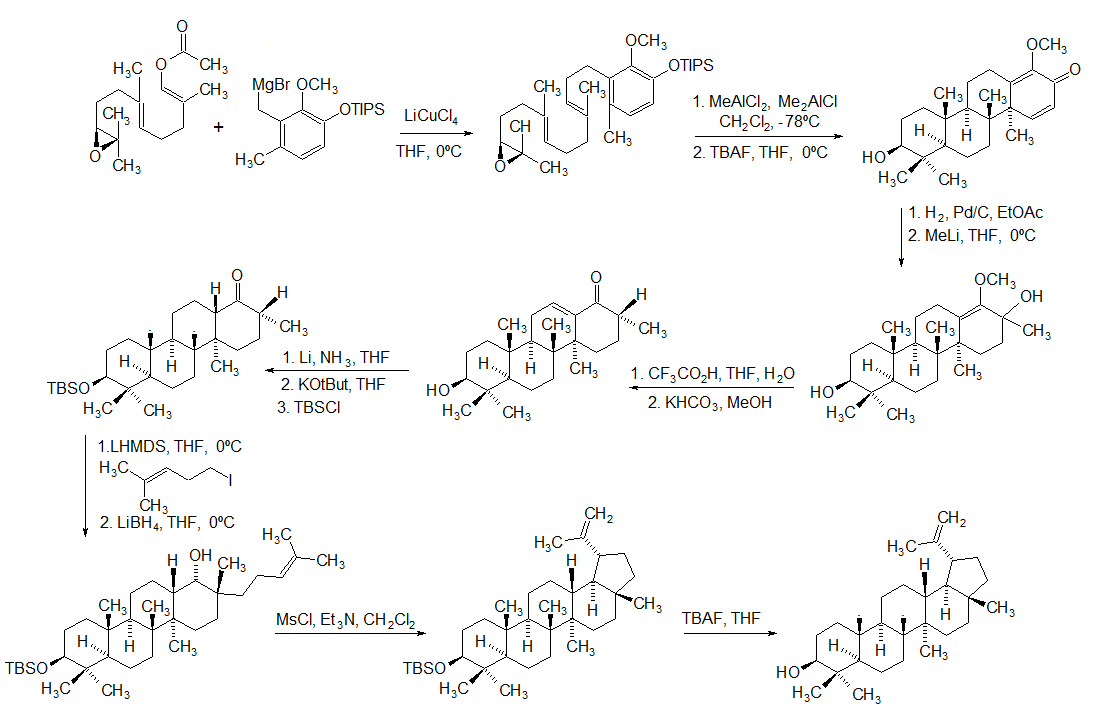
Lupeol
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, '' Acacia visco'' and '' Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucoderma
Vitiligo is a disorder that causes the skin to lose its color. Specific causes are unknown but studies suggest a link to immune system changes. Signs and symptoms The only sign of vitiligo is the presence of pale patchy areas of depigmented skin which tend to occur on the extremities. Some people may experience itching before a new patch occurs. The patches are initially small, but often grow and change shape. When skin lesions occur, they are most prominent on the face, hands and wrists. The loss of skin pigmentation is particularly noticeable around body orifices, such as the mouth, eyes, nostrils, genitalia and umbilicus. Some lesions have increased skin pigment around the edges. Those affected by vitiligo who are stigmatized for their condition may experience depression and similar mood disorders. File:Vitiligo03.jpg, Vitiligo on lighter skin File:Vitiligo1.JPG, Non-segmental vitiligo on dark skin, hand facing up File:Eyelid vitiligo 06.jpg, Non-segmental vitiligo o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutin
Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose). It is a flavonoid found in a wide variety of plants, including citrus. Occurrences Rutin is one of the phenolic compounds found in the invasive plant species, ''Carpobrotus edulis''. Its name comes from the name of ''Ruta graveolens'', a plant that also contains rutin. Various citrus fruit peels contain 32 to 49 mg/g of flavonoids expressed as rutin equivalents. Citrus leaves contain rutin at concentrations of 11 and 7 g/kg in orange and lime trees, respectively. In 2021, Samoan researchers identified rutin in the native plant, ''matalafi'' (''Psychotria insularum''). Metabolism The enzyme quercitrinase found in ''Aspergillus flavus'' is in the rutin catabolic pathway. In food Rutin is a citrus flavonoid glycoside found in many plants, including buckwheat, the leaves and pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperoside
Hyperoside is a chemical compound. It is the 3-''O''-galactoside of quercetin. Natural occurrences Hyperoside has been isolated from ''Drosera rotundifolia'', from the Lamiaceae ''Stachys sp.'' and ''Prunella vulgaris'', from ''Rumex acetosella'', ''Cuscuta chinensis'' seeds, from St John's wort and from ''Camptotheca acuminata''. It is one of the phenolic compounds in the invasive plant ''Carpobrotus edulis'' and contributes to the antibacterial properties of the plant. In ''Rheum nobile'' and '' R. rhaponticum'', it serves as a UV blocker found in the bracts. It is also found in ''Geranium niveum'' and ''Taxillus kaempferi ''Taxillus kaempferi'' () is a parasitic plant A parasitic plant is a plant that derives some or all of its nutritional requirements from another living plant. They make up about 1% of angiosperms and are found in almost every biome. All para ...''.The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Tax ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tannin
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids. The term ''tannin'' (from Anglo-Norman ''tanner'', from Medieval Latin ''tannāre'', from ''tannum'', oak bark) refers to the use of oak and other bark in tanning animal hides into leather. By extension, the term ''tannin'' is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with various macromolecules. The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation (acting as pesticides) and might help in regulating plant growth. The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit, red wine or tea. Likewise, the destruction or modification of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
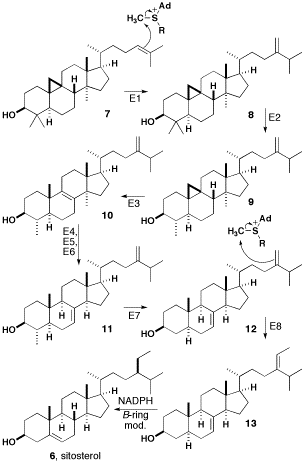
Sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" also describes the conjugate acid, conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic ion, polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |