|
Hammond's Postulate
Hammond's postulate (or alternatively the Hammond–Leffler postulate), is a hypothesis in physical organic chemistry which describes the geometric structure of the transition state in an organic chemical reaction. First proposed by George Hammond in 1955, the postulate states that: If two states, as, for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures. Therefore, the geometric structure of a state can be predicted by comparing its energy to the species neighboring it along the reaction coordinate. For example, in an ''exothermic'' reaction the transition state is closer in energy to the reactants than to the products. Therefore, the transition state will be more geometrically similar to the reactants than to the products. In contrast, however, in an ''endothermic'' reaction the transition s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
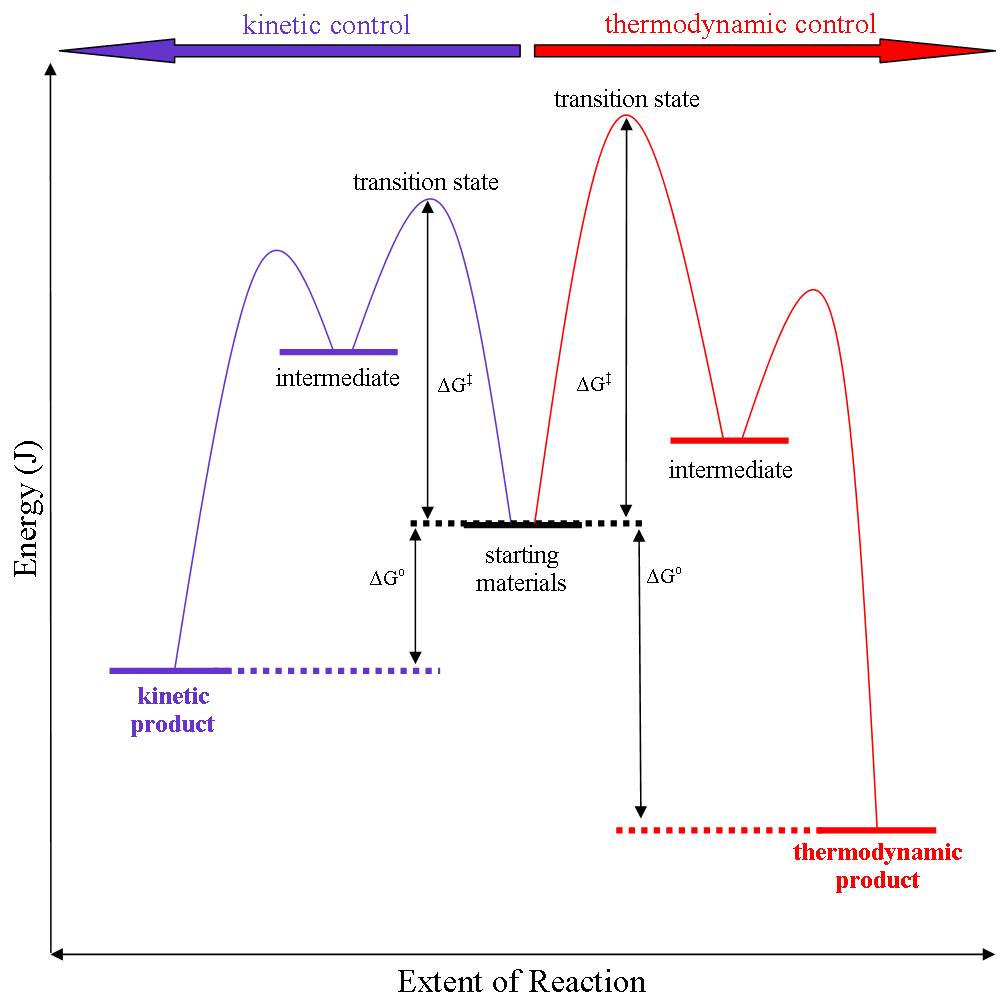
Thermodynamic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner–Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate-limiting Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–volume work, pressure–volume work, that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as G(p,T) = U + pV - TS = H - TS where: * U is the internal energy of the system * H is the enthalpy of the system * S is the entropy of the system * T is the temperature of the system * V is the volume of the system * p is the pressure of the system (which must be equal to that of the surroundings for mechanical equilibrium). The Gibbs free energy change (, measured in joules in International System of Units, SI) is the ''maximum'' amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the Thermodynamic system, system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of ''equilibrium chemistry''. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate-determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Enthalpy Of Reaction
The standard enthalpy of reaction (denoted \Delta H_^\ominus) for a chemical reaction is the difference between total product and total reactant molar enthalpies, calculated for substances in their standard states. The value can be approximately interpreted in terms of the total of the chemical bond energies for bonds broken and bonds formed. For a generic chemical reaction :\nu_ \text + \nu_ \text ~+ ~... \rightarrow \nu_ \text + \nu_ \text ~+ ~... the standard enthalpy of reaction \Delta H_^\ominus is related to the standard enthalpy of formation \Delta_ H^\ominus values of the reactants and products by the following equation: : \Delta H_^\ominus = \sum_ \nu_p\Delta_ H_^ - \sum_ \nu_r\Delta_ H_^ In this equation, \nu_i are the stoichiometric coefficients of each product and reactant. The standard enthalpy of formation, which has been determined for a vast number of substances, is the change of enthalpy during the formation of 1 mole of the substance from its constituen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History The pioneering work of chemical kinetics was done by German chemist Ludwig Wilhelmy in 1850. He experimentally studied the rate of inversion of sucrose and he used integrated rate law for the determination of the reaction kinetics of this reaction. His work was noticed 34 years later by Wilhelm Ostwald. In 1864, Peter Waage and Cato Guldberg published the law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E1 Rxn Coordinate
E1, E01, E.I or E-1 may refer to: Transportation Aircraft * Azcárate E-1, a Mexican sesquiplane trainer * Fokker E.I, a German fighter aircraft * Grumman E-1 Tracer, an American airborne early warning aircraft * Hydra Technologies E1 Gavilán, a hand-launched Mexican unmanned electronic surveillance drone * Junkers E.I, the Idflieg designation for the 1916 Junkers J1 monoplane * LVG E.I, a 1915 German two-seat monoplane * NFW E.I, a 1917 German monoplane fighter * Pfalz E.I, a Morane-Saulnier H monoplane built under licence for Germany * Siemens-Schuckert E.I, a 1915 German single seat monoplane * Standard E-1, a 1917 early American Army fighter aircraft Automobiles * BMW E1, a 1991 and 1993 German electric/hybrid city car concept * BYD e1, a 2019–present Chinese electric city car * Dongfeng Fengguang E1, a 2019–present Chinese electric mini crossover * Haima E1, a 2020–present Chinese electric city car * Roewe E1, a 2012 Chinese electric city car concept later p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |