|
HMG-CoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low Density Lipoprotein
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons (aka ULDL by the overall density naming convention), very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to Cell (biology), cells. LDL is involved in atherosclerosis, a process in which it is oxidized within the walls of Artery, arteries. Overview Lipoproteins transfer lipids (fats) around the body in the extracellular fluid, making fats available to body cells for receptor-mediated endocytosis. Lipoproteins are complex particles composed of multiple proteins, typically 80–100 proteins per particle (organized by a single apolipoprotein B for LDL and the larger particles). A single LDL particle is about 220–275 angstroms in diameter, typically transpor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simvastatin
Simvastatin, sold under the brand name Zocor among others, is a statin, a type of lipid-lowering medication. It is used along with exercise, diet, and weight loss to decrease elevated lipid levels. It is also used to decrease the risk of heart problems in those at high risk. It is taken by mouth. Common side effects include constipation, headaches, and nausea. Serious side effects may include muscle breakdown, liver problems, and increased blood sugar levels. A lower dose may be needed in people with kidney problems. There is evidence of harm to the developing baby when taken during pregnancy and it should not be used by those who are breastfeeding. It is in the statin class of medications and works by decreasing the manufacture of cholesterol by the liver. Simvastatin is made from the fungus ''Aspergillus terreus''. It was patented by Merck in 1980, and came into medical use in 1992. Simvastatin is available as a generic medication, and is on the World Health Organization' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pitavastatin
Pitavastatin (usually as a calcium salt) is a member of the blood cholesterol lowering medication class of statins. Like other statins, it is an inhibitor of HMG-CoA reductase, the enzyme that catalyses the first step of cholesterol synthesis. It was patented in 1987 and approved for medical use in 2003. It is available in Japan, South Korea and in India. In the US, it received FDA approval in 2009. Kowa Pharmaceuticals, a subsidiary of Kowa Company, is the owner of the American patent to pitavastatin. Medical uses Like the other statins, pitavastatin is indicated for hypercholesterolaemia (elevated cholesterol) and for the prevention of cardiovascular disease. A 2009 study of the 104-week LIVES trial found pitavastatin increased HDL cholesterol, especially in patients with HDL lower than 40 mg/dL, who had a 24.6% rise, in addition to greatly reducing LDL cholesterol 31.3%. HDL improved in patients who switched from other statins and rose over time. In the 70-month C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluvastatin
Fluvastatin is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular disease. It was patented in 1982 and approved for medical use in 1994. It is on the World Health Organization's List of Essential Medicines. Adverse effects Adverse effects are comparable to other statins. Common are nausea, indigestion, insomnia and headache. Myalgia (muscle pain), and rarely rhabdomyolysis, characteristic side effects for statins, can also occur. Interactions Contrary to lovastatin, simvastatin and atorvastatin, fluvastatin has no relevant interactions with drugs that inhibit the liver enzyme CYP3A4, and a generally lower potential for interactions than most other statins. Fluconazole, a potent inhibitor of CYP2C9, does increase fluvastatin levels. Pharmacology Mechanism of action Fluvastatin works by blocking the liver enzyme HMG-CoA reductase, which facilitates an important step in cholesterol synthesis. Pharmacodynamics In a Coch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pravastatin
Pravastatin, sold under the brand name Pravachol among others, is a statin medication, used for preventing cardiovascular disease in those at high risk and treating abnormal lipids. It should be used together with diet changes, exercise, and weight loss. It is taken by mouth. Common side effects include joint pain, diarrhea, nausea, headaches, and muscle pains. Serious side effects may include rhabdomyolysis, liver problems, and diabetes. Use during pregnancy may harm the baby. Like all statins, pravastatin works by inhibiting HMG-CoA reductase, an enzyme found in liver that plays a role in producing cholesterol. Pravastatin was patented in 1980 and approved for medical use in 1989. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 34th most commonly prescribed medication in the United States, with more than 17million prescriptions. Medical uses Pravastatin is primarily used for the treatment o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth. Common side effects include joint pain, diarrhea, heartburn, nausea, and muscle pains. Serious side effects may include rhabdomyolysis, liver problems, and diabetes. Use during pregnancy may harm the fetus. Like all statins, atorvastatin works by inhibiting HMG-CoA reductase, an enzyme found in the liver that plays a role in producing cholesterol. Atorvastatin was patented in 1986, and approved for medical use in the United States in 1996. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the most commonly prescribed medication in the United States, with more than 114million prescriptions. Medical uses The primary uses of atorvastatin is for the treatment of dyslipide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
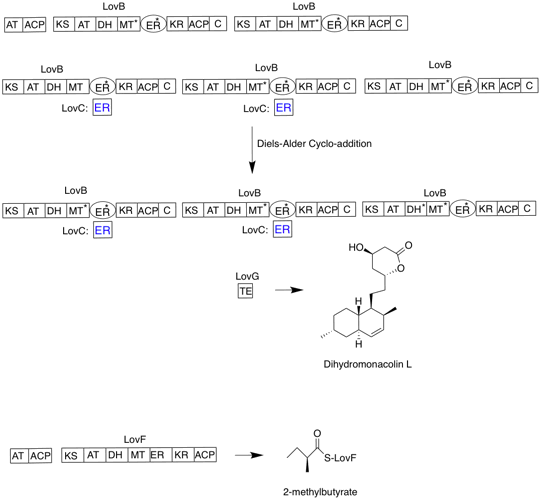
Lovastatin
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat hypercholesterolemia, high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth. Common side effects include diarrhea, constipation, headache, muscles pains, rash, and trouble sleeping. Serious side effects may include liver problems, rhabdomyolysis, muscle breakdown, and kidney failure. Use during pregnancy may harm the baby and use during breastfeeding is not recommended. It works by decreasing the liver's ability to produce cholesterol by blocking the enzyme HMG-CoA reductase. Lovastatin was patented in 1979 and approved for medical use in 1987. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 99th most commonly prescribed medication in the United States, with more than 7mill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rosuvastatin
Rosuvastatin, sold under the brand name Crestor among others, is a statin medication, used to prevent cardiovascular disease in those at high risk and treat abnormal lipids. It is recommended to be used together with dietary changes, exercise, and weight loss. It is taken by mouth. Common side effects include abdominal pain, nausea, headaches, and muscle pains. Serious side effects may include rhabdomyolysis, liver problems, and diabetes. Use during pregnancy may harm the baby. Like all statins, rosuvastatin works by inhibiting HMG-CoA reductase, an enzyme found in the liver that plays a role in producing cholesterol. Rosuvastatin was patented in 1991, and approved for medical use in the United States in 2003. It is available as a generic medication. In 2020, it was the seventeenth most commonly prescribed medication in the United States, with more than 29million prescriptions. There have been criticisms of rosuvastatin having worse adverse effects despite effectively reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heart Disease
Cardiovascular disease (CVD) is a class of diseases that involve the heart or blood vessels. CVD includes coronary artery diseases (CAD) such as angina and myocardial infarction (commonly known as a heart attack). Other CVDs include stroke, heart failure, hypertensive heart disease, rheumatic heart disease, cardiomyopathy, abnormal heart rhythms, congenital heart disease, valvular heart disease, carditis, aortic aneurysms, peripheral artery disease, thromboembolic disease, and venous thrombosis. The underlying mechanisms vary depending on the disease. It is estimated that dietary risk factors are associated with 53% of CVD deaths. Coronary artery disease, stroke, and peripheral artery disease involve atherosclerosis. This may be caused by high blood pressure, smoking, diabetes mellitus, lack of exercise, obesity, high blood cholesterol, poor diet, excessive alcohol consumption, and poor sleep, among other things. High blood pressure is estimated to account for approximatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG-CoA Reductase Inhibitor
Statins, also known as HMG-CoA reductase inhibitors, are a class of lipid-lowering medications that reduce illness and mortality in those who are at high risk of cardiovascular disease. They are the most common cholesterol-lowering drugs. Low-density lipoprotein (LDL) carriers of cholesterol play a key role in the development of atherosclerosis and coronary heart disease via the mechanisms described by the lipid hypothesis. Statins are effective in lowering LDL cholesterol and so are widely used for primary prevention in people at high risk of cardiovascular disease, as well as in secondary prevention for those who have developed cardiovascular disease. Side effects of statins include muscle pain, increased risk of diabetes mellitus, and abnormal blood levels of liver enzymes. Additionally, they have rare but severe adverse effects, particularly muscle damage. They inhibit the enzyme HMG-CoA reductase which plays a central role in the production of cholesterol. High cholestero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Pharmaceutical drugs are often classified into drug classes—groups of related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)

