|
Glossostemon Bruguieri
''Glossostemon bruguieri'' or ''Dombeya arabica'' is a species of flowering plant in the family Malvaceae. It is a shrub with thick long tapering dark colored roots with 70–100 cm in length and 5–8 cm in breadth, found in Yemen, Iran, Iraq, Egypt, Saudi Arabia, Turkey and Morocco. The dried peeled roots of ''G. bruguieri'' are called in Egypt and Arab countries ( ar, مُغات ). The roots are commonly used in traditional medicine for many nutritional and medicinal values. Chemical composition Starch is the main component of the dried peeled roots with 54.5–62.4% (differs according to the climatic region of cultivation) while protein represents 4.5–8.3%, half of which is aspartic acid. Roots contain high amounts of non-starch polysaccharides including dietary fibers, pectin and up to 27% of mucilage. Calcium, magnesium and iron are the main minerals of the roots. Minor amounts of zinc, manganese and copper have also been found. Tatakin (4-methoxyisoscutell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
René Louiche Desfontaines
René Louiche Desfontaines (14 February 1750 – 16 November 1833) was a French botanist. Desfontaines was born near Tremblay, Ille-et-Vilaine , Tremblay in Brittany. He attended the Collège de Rennes and in 1773 went to Paris to study medicine. His interest in botany originated from lectures at the Jardin des Plantes given by Louis Guillaume Lemonnier. He excelled in his new interest and was elected to the French Academy of Sciences in 1783. He was also a member of the Académie Nationale de Médecine. Desfontaines spent two years in Tunisia and Algeria, returning with a large collection of plants. He wrote ''Flora Atlantica'' (1798–1799, 2 vols), which included 300 genera new to science. In addition, he worked also on ornithology, and presented the findings of his expeditions to Africa for one of the ''Memoires de L'Académie Royale des Sciences''. Although the ''Mémoire'' corresponds to the year 1787, it was not published until 1789 by L'Imprimerie Royal as part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxsalen
Methoxsalen, sold under the brand name Oxsoralen among others, is a medication used to treat psoriasis, eczema, vitiligo, and some cutaneous lymphomas in conjunction with exposing the skin to ultraviolet (UVA) light from lamps or sunlight. Methoxsalen modifies the way skin cells receive the UVA radiation, allegedly clearing up the disease. Levels of individual patient PUVA exposure were originally determined using the Fitzpatrick scale. The scale was developed after patients demonstrated symptoms of phototoxicity after oral ingestion of methoxsalen followed by PUVA therapy. Chemically, methoxsalen belongs to a class of organic natural molecules known as furanocoumarins. They consist of coumarin annulated with furan. It can also be injected and used topically. Natural sources In 1947, methoxsalen was isolated (under the name "ammoidin") from the plant ''Ammi majus'', bishop's weed. In 1970, Nielsen extracted 8-methoxypsoralen from four species of the genus '' Heracleum'' in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhamnose
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as ''Pseudomonas aeruginosa'' and ''Helicobacter pylori''. Rhamnose can be isolated from Buckthorn (''Rhamnus''), poison sumac, and plants in the genus ''Uncaria''. Rhamnose is also produced by microalgae belonging to class Bacillariophyceae (diatoms). Rhamnose is commonly bound to other sugars in nature. It is a common glycone component of glycosides from many plants. Rhamnose is also a component of the outer cell membrane of acid-fast bacteria in the ''Mycobacterium'' genus, which includes the organism that cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucuronic Acid
Glucuronic acid (from Greek γλεῦκος "''wine, must''" and οὖρον "''urine''") is a uronic acid that was first isolated from urine (hence the name). It is found in many gums such as gum arabic (c. 18%), xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals. Properties Glucuronic acid is a sugar acid derived from glucose, with its sixth carbon atom oxidized to a carboxylic acid. In living beings, this primary oxidation occurs with UDP-α-D-glucose (UDPG), not with the free sugar. Glucuronic acid, like its precursor glucose, can exist as a linear (carboxo-) aldohexose ( 60,000 are too large for renal excretion and will be excreted with bile into the intestine. Neonates are deficient in this conjugating system, making them particularly vulnerable to drugs such as chloramphenicol, which is inactivated by the addition of glucuronic acid, resulting in gray baby syndrome. Bilirubin is excreted in the bile as bilirubin dig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arabinose
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group. For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.The D/L nomenclature does not refer to the molecule's optical rotation properties but to its structural analogy to glyceraldehyde. However, L-arabinose is in fact more common than D-arabinose in nature and is found in nature as a component of biopolymers such as hemicellulose and pectin. The L-arabinose operon, also known as the araBAD operon, has been the subject of much biomolecular research. The operon directs the catabolism of arabinose in ''E. coli'', and it is dynamically activated in the presence of arabinose and the absence of glucose. A classic method for the organic synthesis of arabinose from glucose is the Wohl degradation. : Etymology Arabinose gets its name from gum arabic, from which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biflavone
Biflavonoids are a type of flavonoids with the general formula scheme (C6-C3-C6)2. Examples * Amentoflavone (bis-apigenin coupled at 8 and 3' positions) * Lophirone L and lophirone M found in ''Lophira alata'' * Sulcatone A, a naturally occurring biflavonoid isolated from '' Ouratea sulcata''. Extracts of the leaves of this plant, used with and with other plant's extracts, are used in many African countries to treat some infections such as upper tract respiratory infections, dysenteria, diarrhoea and toothache. Positive antimicrobial activity has been shown in-vitro against ''Staphylococcus aureus'' and ''Bacillus subtilis''. ''Escherichia coli'' showed to be resistant in the same study. * Hinokiflavone, a cytotoxic biflavonoid from ''Toxicodendron succedaneum'', ''Juniperus sp.'', or ''Chamaecyparis obtusa'' (hinoki). * Leaflets of ''Cycas circinalis'' and '' C. revoluta'' contain biflavonoids such as (2''S'', 2′′''S'')-2,3,2′′,3′′-tetrahydro-4′,4′′′-di-''O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-amyrin
The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee. A study demonstrated that α,β-amyrin exhibits long-lasting antinociceptive and anti-inflammatory properties in 2 models of persistent nociception via activation of the cannabinoid receptors CB1 and CB2 and by inhibiting the production of cytokines and expression of NF-κB, CREB and cyclooxygenase 2 Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) (The HUGO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campesterol
Campesterol is a phytosterol whose chemical structure is similar to that of cholesterol, and is one of the ingredients for E number E499. Natural occurrences Many vegetables, fruits, nuts, and seeds contain campesterol, but in low concentrations. Banana, pomegranate, pepper, coffee, grapefruit, cucumber, onion, oat, potato, and lemon grass (citronella) are few examples of common sources containing campesterol at roughly 1–7 mg/100 g of the edible portion. In contrast, canola and corn oils contain as much as 16–100 mg/100 g. Levels are variable and are influenced by geography and growing environment. In addition, different strains have different levels of plant sterols. A number of new genetic strains are currently being engineered with the goal of producing varieties high in campesterol and other plant sterols. It is also found in dandelion coffee. It is so named because it was first isolated from the rapeseed (''Brassica campestris''). It is thought to have a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stigmasterol
Stigmasterol – a plant sterol (''phytosterol'') – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes. In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol. Discovery Once called ''Wulzen factor'' in the mid-20th century, stigmasterol was discovered by the University of California physiologist Rosalind Wulzen (born 1886). Natural occurrences Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of numerous plants, such as soybean, calabar bean, and rape seed, and in herbs used in herbalism practices, including the Chinese herbs ''Ophiopogon japonicus'' (Mai men dong), in ''Mirabilis jalapa''. Stigmasterol is a constituent of various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactivate stig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
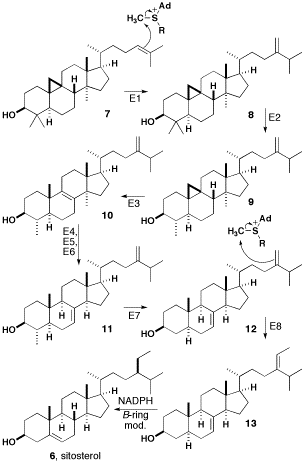
β-sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytosterols
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanols. More than 250 sterols and related compounds have been identified. Free phytosterols extracted from oils are insoluble in water, relatively insoluble in oil, and soluble in alcohols. Phytosterol-enriched foods and dietary supplements have been marketed for decades. Despite well-documented LDL cholesterol-lowering effects from long-term consumption of phytosterols, there is insufficient evidence for an effect on cardiovascular diseases, fasting blood sugar, glycated hemoglobin, or overall mortality rate. Structure They have a fused polycyclic structure and vary in carbon side chains and / or presence or absence of a double bond (saturation). They are divided into 4,4-dimethyl phytosterols, 4-monomethyl phytosterols, and 4-desmethyl phytosterols based on the location of methyl groups at the carbon-4 position. Sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |