|
Gibbs–Donnan Effect
The Gibbs–Donnan effect (also known as the Donnan's effect, Donnan law, Donnan equilibrium, or Gibbs–Donnan equilibrium) is a name for the behaviour of charged particles near a semi-permeable membrane that sometimes fail to distribute evenly across the two sides of the membrane. The usual cause is the presence of a different charged substance that is unable to pass through the membrane and thus creates an uneven electrical charge. For example, the large anionic proteins in blood plasma are not permeable to capillary walls. Because small cations are attracted, but are not bound to the proteins, small anions will cross capillary walls away from the anionic proteins more readily than small cations. Thus, some ionic species can pass through the barrier while others cannot. The solutions may be gels or colloids as well as solutions of electrolytes, and as such the phase boundary between gels, or a gel and a liquid, can also act as a selective barrier. The electric potential arisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
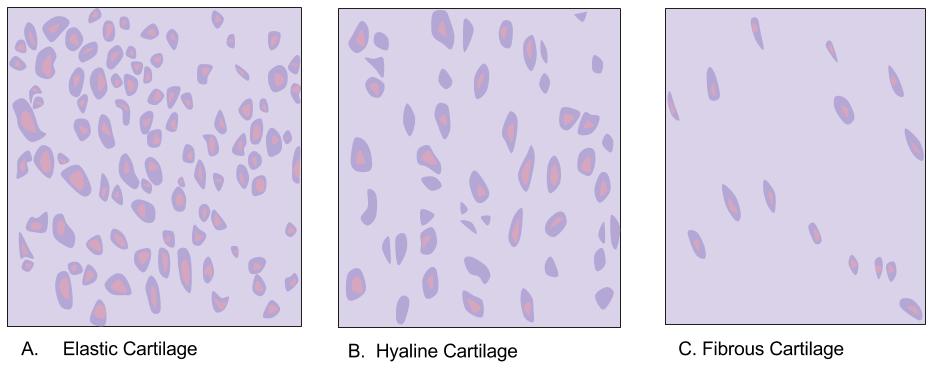
Cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized cells called chondrocytes that produce a large amount of collagenous extracellular matrix, abundant ground substance that is rich in pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion Equilibrium
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Layer (biology)
In biological systems, a double layer is the surface where two different phases of matter are in contact. Biological double layers are much like their interfacial counterparts, but with several notable distinctions. The surface of biological cells carry many different types of chemical groups, each with a different dissociation constant, causing them to have varying electric charges at a physiological pH. This indicates that biosurfaces are chemically heterogeneous. This biospecific feature is typical for all biosurfaces, including proteins, macromolecules and biological cells. In certain organisms, cells are covered with the glycocalyx layer, which can be modeled as a polyelectrolyte layer with a volume spread electric charge. This means that the notion of a surface charge is located on certain flat surfaces. This does not apply; instead, the cell surface is a finite thickness polyelectrolyte layer with a volume charge. At equilibrium, the relationship between these polyelec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nernst Equation
In electrochemistry, the Nernst equation is a Thermodynamics#Chemical thermodynamics, chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (half-cell or electrochemical cell, full cell reaction) from the standard electrode potential, Thermodynamic temperature, absolute temperature, the number of electrons involved in the redox, redox reaction, and Thermodynamic activity, activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation respectively. It was named after Walther Nernst, a German physical chemist who formulated the equation. Expression General form with chemical activities When an oxidizer () accepts a number ''z'' of electrons () to be converted in its reduced form (), the half-reaction is expressed as: : + ''z'' → The reaction quotient ('), also often called the ion activity product (''IAP''), is the ratio between the chemical activity, chemical activities (' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV. All animal cells are surrounded by a membrane composed of a lipid bilayer with proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. Transmembrane proteins, also known as ion transporter or ion pump proteins, actively push ions across the membrane and establish concentration gradi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracranial Pressure
Intracranial pressure (ICP) is the pressure exerted by fluids such as cerebrospinal fluid (CSF) inside the skull and on the brain tissue. ICP is measured in millimeters of mercury (mmHg) and at rest, is normally 7–15 Millimeter of mercury, mmHg for a Supine position, supine adult. The body has various mechanisms by which it keeps the ICP stable, with CSF pressures varying by about 1 mmHg in normal adults through shifts in production and absorption of CSF. Changes in ICP are attributed to volume changes in one or more of the constituents contained in the cranium. CSF pressure has been shown to be influenced by abrupt changes in intrathoracic pressure during coughing (which is induced by contraction of the diaphragm and abdominal wall muscles, the latter of which also increases intra-abdominal pressure), the valsalva maneuver, and communication with the vasculature (venous and arterial systems). Intracranial hypertension (IH), also called increased ICP (IICP) or raised intracrani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerebral Edema
Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain. This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels. Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, seizures, drowsiness, visual disturbances, dizziness, and in severe cases, coma and death. Cerebral edema is commonly seen in a variety of brain injuries including ischemic stroke, subarachnoid hemorrhage, traumatic brain injury, subdural, epidural, or intracerebral hematoma, hydrocephalus, brain cancer, brain infections, low blood sodium levels, high altitude, and acute liver failure. Diagnosis is based on symptoms and physical examination findings and confirmed by serial neuroimaging ( computed tomography scans and magnetic resonance imaging). The treatment of cerebral edema depends on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nervous Tissue
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons. Nervous tissue is made up of different types of neurons, all of which have an axon. An axon is the long stem-like part of the cell that sends action potentials to the next cell. Bundles of axons make up the nerves in the PNS and tracts in the CNS. Functions of the nervous system are sensory input, integration, control of muscles and glands, homeostasis, and mental activity. Structure Nervous tissue is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity (chemistry)
In chemical thermodynamics, activity (symbol ) is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution. The term "activity" in this sense was coined by the American chemist Gilbert N. Lewis in 1907. By convention, activity is treated as a dimensionless quantity, although its value depends on customary choices of standard state for the species. The activity of pure substances in condensed phases (solid or liquids) is normally taken as unity (the number 1). Activity depends on temperature, pressure and composition of the mixture, among other things. For gases, the activity is the effective partial pressure, and is usually referred to as fugacity. The difference between activity and other measures of concentration arises because the interactions between different types of molecules in non-ideal gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |