|
Gallocyanin Stain
The gallocyanin stain, also known as the gallocyanin-chromalum stain, is a stain of the oxazine group for total nucleic acids. It is prepared from gallocyanin and is an ideal method for numerous slides that need to be stained serially, equivalently, and reproducible. Structures containing basophilic compounds take on a bluish color. History It has been known since the early work of Einarson (1932) that the gallocyanin dye worked well for nucleotide constituents. Gersch and colleagues at Chicago are often credited with the earliest efforts of using gallocyanin for staining. Sandritter demonstrated that a stoichiometric relationship occurs between intensity of staining and quantity of nucleic acid present. Function Its method of binding and specificity are still not completely known. However, it is thought that gallocyanin-Cr(H2O)4 selectively binds to nucleic acid phosphate groups, particularly within a pH range In chemistry, pH (), historically denoting "potential o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallocyanin
Gallocyanin is a chemical compound classified as a phenoxazine dye. In combination with certain metals, it is used to prepare gallocyanin stains that are used in identifying nucleic acid Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...s. References Phenoxazines Oxazine dyes Carboxylic acids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazine
Oxazines are heterocyclic compounds containing one oxygen and one nitrogen atom in a doubly unsaturated six-membered ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds. By extension, the derivatives are also referred to as oxazines; examples include ifosfamide and morpholine (tetrahydro-1,4-oxazine). A commercially available dihydro-1,3-oxazine is a reagent in the Meyers synthesis of aldehydes. Fluorescent dyes such as Nile red and Nile blue are based on the aromatic benzophenoxazine. Cinnabarine and cinnabaric acid are two naturally occurring dioxazines, being derived from biodegradation of tryptophan. Dioxazines Dioxazines are pentacyclic compounds consisting of two oxazine subunits. A commercially important example is the pigment pigment violet 23. Benzoxazines Benzoxazines are bicyclic compounds formed by the ring fusion of a benzene ring with an oxazine. Polybenzoxazines are a class of polymers formed by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA. Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and make up the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded information is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallocyanin
Gallocyanin is a chemical compound classified as a phenoxazine dye. In combination with certain metals, it is used to prepare gallocyanin stains that are used in identifying nucleic acid Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...s. References Phenoxazines Oxazine dyes Carboxylic acids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
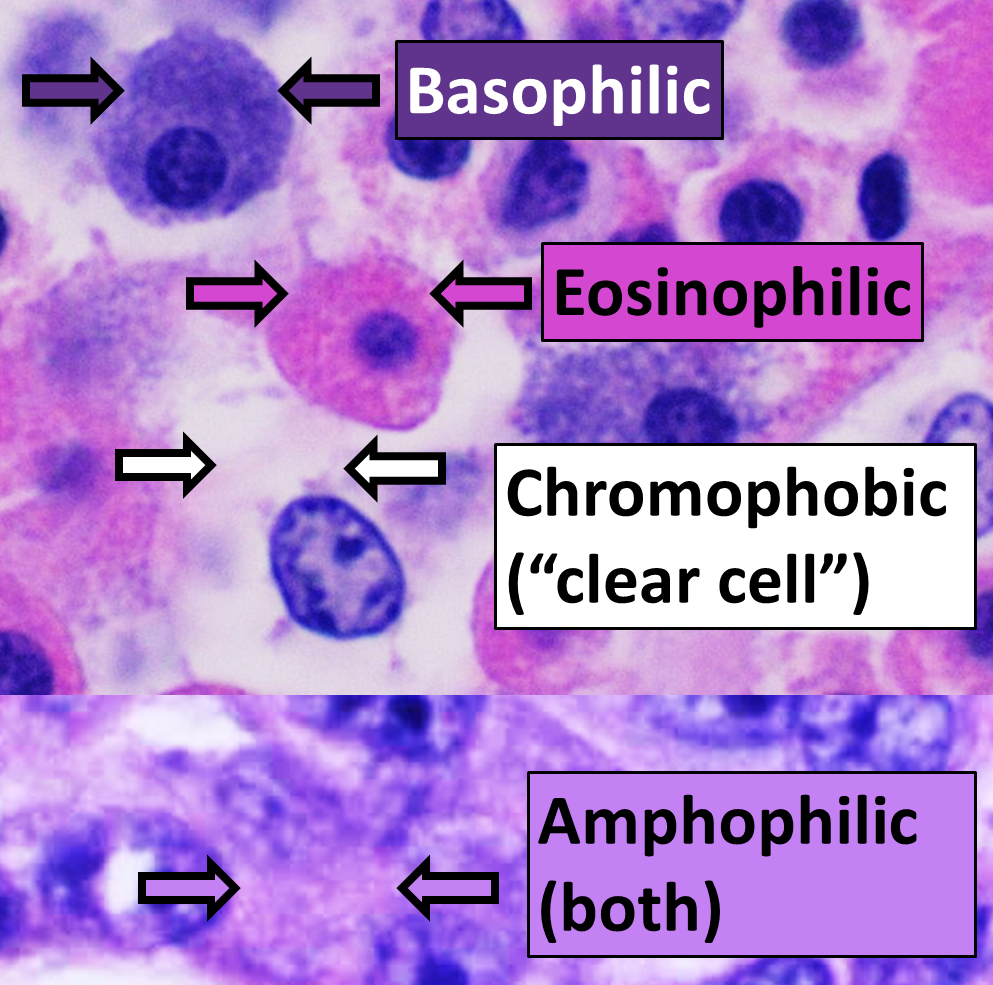
Basophilic Compound
Basophilic is a technical term used by pathologists. It describes the appearance of cells, tissues and cellular structures as seen through the microscope after a histological section has been stained with a basic dye. The most common such dye is haematoxylin. The name basophilic refers to the characteristic of these structures to be stained very well by basic dyes. This can be explained by their charges. Basic dyes are cationic, i.e. contain positive charges, and thus they stain anionic structures (i.e. structures containing negative charges), such as the phosphate backbone of DNA in the cell nucleus and ribosomes. "Basophils" are cells that "love" (from greek "-phil") basic dyes, for example haematoxylin, azure and methylene blue Methylthioninium chloride, commonly called methylene blue, is a salt used as a dye and as a medication. Methylene blue is a thiazine dye. As a medication, it is mainly used to treat methemoglobinemia by converting the ferric iron in hemogl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combustion. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |