|
Grieco Three-component Condensation
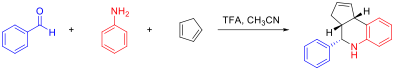
The Grieco three-component condensation is an organic chemistry reaction that produces nitrogen-containing six-member heterocycles via a multi-component reaction of an aldehyde, a nitrogen component, such as aniline, and an electron-rich alkene. The reaction is catalyzed by trifluoroacetic acid or Lewis acids such as ytterbium trifluoromethanesulfonate (Yb(OTf)3). The reaction is named for Paul Grieco, who first reported it in 1985. In the original paper the nitrogen component were benzylamine, methyl amine or ammonium chloride, the reaction now also include anilines, similar to the earlier Povarov reaction. The reaction process involves the formation of an aryl immonium ion intermediate followed by an aza Diels-Alder reaction with an alkene. Imines are electron-poor, and thus usually function as the dienophile. Here, however, the alkene is electron-rich, so it reacts well with the immonium diene in an Inverse electron-demand Diels–Alder reaction. Researchers have extended t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grieco Three-component Coupling
The Grieco three-component condensation is an organic chemistry reaction that produces nitrogen-containing six-member heterocycles via a multi-component reaction of an aldehyde, a nitrogen component, such as aniline, and an electron-rich alkene. The reaction is catalyzed by trifluoroacetic acid or Lewis acids such as ytterbium trifluoromethanesulfonate (Yb(OTf)3). The reaction is named for Paul Grieco, who first reported it in 1985. In the original paper the nitrogen component were benzylamine, methyl amine or ammonium chloride, the reaction now also include anilines, similar to the earlier Povarov reaction. The reaction process involves the formation of an aryl immonium ion intermediate followed by an aza Diels-Alder reaction with an alkene. Imines are electron-poor, and thus usually function as the dienophile. Here, however, the alkene is electron-rich, so it reacts well with the immonium diene in an Inverse electron-demand Diels–Alder reaction. Researchers have extended t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Povarov Reaction
The Povarov reaction is an organic reaction described as a formal cycloaddition between an aromatic imine and an alkene. The imine in this organic reaction is a condensation reaction product from an aniline type compound and a benzaldehyde type compound. The alkene must be electron rich which means that functional groups attached to the alkene must be able to donate electrons. Such alkenes are enol ethers and enamines. The reaction product in the original Povarov reaction is a quinoline. Because the reactions can be carried out with the three components premixed in one reactor it is an example of a multi-component reaction. : Reaction mechanism The reaction mechanism for the Povarov reaction to the quinoline is outlined in ''scheme 1''. In step one aniline and benzaldehyde react to the Schiff base in a condensation reaction. The Povarov reaction requires a Lewis acid such as boron trifluoride to activate the imine for an electrophilic addition of the activated alkene. This reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery involves the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, stry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia. Scandium is present in most of the deposits of rare-earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide. Because of the low availability and difficulties in the preparation of metallic scandium, which was first done in 1937, applications for scandium were not developed until the 1970s, when the positive effects of scandium on aluminium alloys were discovered, and its use in such alloys remains its only major application. The global trade of scandium oxide is 15–20 tonnes per year. The properties of scandium compounds are intermediate between those of aluminium and yttrium. A diagonal relationship exists betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combinatorial Chemistry
Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number (tens to thousands or even millions) of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides. Strategies that allow identification of useful components of the libraries are also part of combinatorial chemistry. The methods used in combinatorial chemistry are applied outside chemistry, too. History Combinatorial chemistry had been invented by Furka Á (Eötvös Loránd University Budapest Hungary) who described the principle of it, the combinatorial synthesis and a deconvolution procedure in a document that was notarized in 1982.Furka Á. Tanulmány, gyógyászatilag hasznosítható peptidek szisztematikus felkutatásának lehetőségéről (and Study on the possibility of sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inverse Electron-demand Diels–Alder Reaction
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder (or DA) reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right. DAINV reactions often involve heteroatoms, and can be used to form heterocyclic compounds. This makes the DAINV reaction particularly useful in natural product syntheses, where the target compounds often contain heterocycles. Recently, the DAINV reaction has been used to synthesize a drug transport system which targets prostate cancer. History The Diels–Alder reaction was first reported in 1928 by Otto Diels and Kurt Alder; they were awarded the Nobel Prize in chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aza Diels-Alder Reaction
Aza or AZA may refer to: Places *Aza, Azerbaijan, a village and municipality *Azadkənd, Nakhchivan or Lower Aza, Azerbaijan *Aza, medieval name of Haza, Province of Burgos, Spain *Aźa, a Tibetan name for the Tuyuhun kingdom *Aza, a Hebrew romanization for Gaza City or Gaza Strip People *Aza (given name) *Aza of Mannea, king, reigned c. 710–700 BC *Alejandro De Aza (born 1984), Dominican baseball outfielder *Vital Aza (1851–1912), Spanish author, playwright, poet and satirist Other uses *Aza (Kanji: 字), a village or town section in the Japanese addressing system * Azelanic acid, an organic acid used for medical treatment of acne and as whitening agent Abbreviations or initialisms * Azathioprine, an immunosuppressant medication * Association of Zoos and Aquariums (formerly named "American Zoo and Aquarium Association") * Aleph Zadik Aleph, a Jewish youth group that is part of BBYO * ISO 639-3 aza for Azha language, a language of China * ICAO code for Alitalia, an Italian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Grieco
Paul Grieco is an organic chemist at Montana State University. His research focuses on the total synthesis of natural products and the study of solvent effects in various organic reactions. He has received several awards for his work, including the American Chemical Society (ACS) Arthur C. Cope Scholar Award in 1990 and the ACS Award for Creative Work in Synthetic Organic Chemistry in 1991. Among his contributions are two name reaction A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include ...s: the Grieco elimination and the Grieco three-component condensation. References External linksWebsiteMontana State University Year of birth missing (living people) Living people 21st-century American chemists Organic chemists Montana State University faculty {{US-chemist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanism Of The Grieco Three-component Coupling
Mechanism may refer to: *Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission * Mechanism (biology), explaining how a feature is created * Mechanism (philosophy), a theory that all natural phenomena can be explained by physical causes *Mechanism (sociology), a theory that all social phenomena can be explained by the existence of a deterministic mechanism * "The Mechanism", song by Disclosure * ''The Mechanism'' (TV series), a Netflix TV series See also *Machine * Machine (mechanical) * Linkage (mechanical) * Mechanism design, the art of designing rules of a game to achieve a specific outcome * Mechanism of action, the means by which a drug exerts its biological effects * Defence mechanism, unconscious mechanisms aimed at reducing anxiety *Reaction mechanism In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |