|
Grape Reaction Product
The grape reaction product (GRP, GRP1 or 2-S- glutathionyl caftaric acid) is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions. Determining its concentration in wine is possible by mass spectrometry. S-Glutathionyl caftaric acid is itself oxidizable. It is not a substrate for grape polyphenol oxidase, but laccase from ''Botrytis cinerea'' can use it to form GRP2. Related molecules Other related molecules are ''trans''-caffeoyltartrate derivatives like GRP o-quinone and 2,5-di-S-glutathionyl cafteoyl tartrate (GRP2) or adducts with anthocyanidins. See also * Phenolic compounds in wine The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caftaric Acid
Caftaric acid is a non-flavonoid phenolic compound. It is found in the juice of grapes (''Vitis vinifera'') and impacts the color of white wine. It is an esterified phenolic acid, composed of caffeic acid, a hydroxycinnamate produced by plants, and tartaric acid, the principal organic acid found in grape berries. As such, caftaric acid is found in all grape juices and wines. During alcoholic and malolactic fermentation, the ester can be enzymatically hydrolysed, releasing the two constituents. Caffeic acid is susceptible to chemical oxidation, and subsequent redox reactions involving caffeic acid can contribute to wine browning over time, and the straw-gold color that can develop in some white wines after bottling. Aside from wine, it is abundantly present in raisins. It also occurs in ''Cichorium intybus'' (common chicory) and is one of the bioactive components of ''Echinacea purpurea'' (Eastern purple coneflower). Caftaric acid has a good bioavailability when fed in rats. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grape Must
Must (from the Latin ''vinum mustum'', "young wine") is freshly crushed fruit juice (usually grape juice) that contains the skins, seeds, and stems of the fruit. The solid portion of the must is called pomace and typically makes up 7–23% of the total weight of the must. Making must is the first step in winemaking. Because of its high glucose content, typically between 10 and 15%, must is also used as a sweetener in a variety of cuisines. Unlike commercially sold grape juice, which is filtered and pasteurized, must is thick with particulate matter, opaque, and comes in various shades of brown and purple. Winemaking The length of time the pomace stays in the juice is critical for the final character of the wine. When the winemaker judges the time to be right, the juice is drained off the pomace, which is then pressed to extract the juice retained by the matrix. Yeast is added to the juice to begin the fermentation, while the pomace is often returned to the vineyard or orchard for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphenol Oxidase
Polyphenol oxidase (PPO; also polyphenol oxidase i, chloroplastic), an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule. PPO may accept monophenols and/or ''o''-diphenols as substrates. The enzyme works by catalyzing the ''o''-hydroxylation of monophenol molecules in which the benzene ring contains a single hydroxyl substituent to ''o''-diphenols (phenol molecules containing two hydroxyl substituents at the 1, 2 positions, with no carbon between). It can also further catalyse the oxidation of ''o''-diphenols to produce ''o''-quinones. PPO catalyses the rapid polymerization of ''o''-quinones to produce black, brown or red pigments (polyphenols) that cause fruit browning. The amino acid tyrosine contains a single phenolic ring that may be oxidised by the action of PPOs to form ''o''-quinone. Hence, PPOs may also be referred to as tyrosinases. Common foods producing the enzyme include mushrooms (''Agaricus bisporus''), appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laccase
Laccases () are multicopper oxidases found in plants, fungi, and bacteria. Laccases oxidize a variety of phenolic substrates, performing one-electron oxidations, leading to crosslinking. For example, laccases play a role in the formation of lignin by promoting the oxidative coupling of monolignols, a family of naturally occurring phenols. Other laccases, such as those produced by the fungus ''Pleurotus ostreatus'', play a role in the degradation of lignin, and can therefore be classed as lignin-modifying enzymes. Other laccases produced by fungi can facilitate the biosynthesis of melanin pigments. Laccases catalyze ring cleavage of aromatic compounds. Laccase was first studied by Hikorokuro Yoshida in 1883 and then by Gabriel Bertrand in 1894 in the sap of the Japanese lacquer tree, where it helps to form lacquer, hence the name laccase. Active site The active site consists of four copper centers, which adopt structures classified as type I, type II, and type III. A tric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Botrytis Cinerea
''Botrytis cinerea'' is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes. In viticulture, it is commonly known as "botrytis bunch rot"; in horticulture, it is usually called "grey mould" or "gray mold". The fungus gives rise to two different kinds of infections on grapes. The first, grey rot, is the result of consistently wet or humid conditions, and typically results in the loss of the affected bunches. The second, noble rot, occurs when drier conditions follow wetter, and can result in distinctive sweet dessert wines, such as Sauternes (wine), Sauternes or the Aszú of Tokaji/Grasă de Cotnari. The species name ''Botrytis cinerea'' is derived from the Latin for "grapes like ashes"; although poetic, the "grapes" refers to the bunching of the fungal spores on their Conidium, conidiophores, and "ashes" just refers to the greyish colour of the spores ''en masse''. The fungus is usually referred to by its anamorph (asexual form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-quinone
1,2-Benzoquinone, also called ''ortho''-benzoquinone, is an organic compound with formula . It is one of the two isomers of quinone, the other being 1,4-Benzoquinone, 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivative (chemistry), derivatives which are known. Structure The molecule has C symmetry. X-ray crystallography shows that the double bonds are localized, with alternatingly long and short C-C distances within the ring. The C=O distances of 1.21 Å are characteristic of ketones. Preparation and occurrence 1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution or by ortho oxidation of a phenol. It is a precursor to melanin. A strain of the bacterium ''Pseudomonas mendocina'' metabolism, metabolises benzoic acid, yielding 1,2-benzoquinone via catechol. References {{D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−. The main forms of tartrates used commercially are pure crystalline tartaric acid used as an acidulant in non-alcoholic drinks and foods, cream of tartar used in baking, and Rochelle salt, commonly used in electroplating solutions. As food additives As food additives, tartrates are used as antioxidants, acidity regulators, and emulsifiers. Examples include *sodium tartrates ( E335) **monosodium tartrate **sodium tartrate **sodium ammonium tartrate the compound through which Louis Pasteur discovered chirality *potassium tartrates ( E336) **potassium bitartrate (monopotassium tartrate, cream of tartar) **potassium tartrate *potassium sodium tartrate ( E337) *calcium tartrate ( E354, used as emulsifier) * stearyl tartrate ( E483, used as emulsifier) In wine In wine, tartrates are the harmless crystalline depo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthocyanidin
Anthocyanidins are common plant pigments, the sugar-free counterparts of anthocyanins. They are based on the flavylium cation, an oxonium ion, with various groups substituted for its hydrogen atoms. They generally change color from red through purple, blue, and bluish green as a function of pH. Anthocyanidins are an important subclass of the polymethine dyes and flavonoids. The flavylium cation is a chromenylium cation with a phenyl group substituted in position 2; and chromenylium (also called benzopyrylium) is a bicyclic version of pyrylium. The positive charge can move around the molecule. At least 31 monomeric anthocyanidins have been properly identified in living organisms, mostly as the core components of anthocyanins. The latter are responsible for the red, purple, blue, or black color of many fruits (like grapes and blueberries), flowers (like roses), leaves (like purple cabbage), and even tubers (like radishes and purple yams). They are also found in some animals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
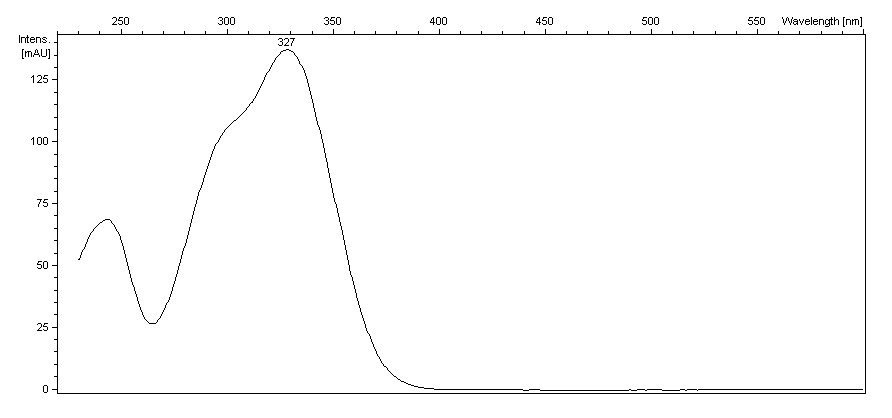
GRP UV Visible Spectrum , in Brazil
{{disambiguation ...
GRP may refer to: Biochemistry * Gastrin-releasing peptide * Grp78, Grp94, Grp170, glucose-regulated proteins * Grape reaction product Mathematics * Grp, the Category of groups Technology and materials * Glass-reinforced-plastic, also known as Fiberglass, or Fibreglass. * Gentoo Reference Platform Transport * Grove Park railway station, London, National Rail station code Other uses * Government resource planning * US Grasslands Reserve Program * Gross rating point * Gross regional product * GRP Records, an American jazz label * Gurupi Airport Comte. Jacinto Nunes Airport is the airport serving Gurupi, Brazil. It is operated by contract by Infraero. History On June 2, 2023 the Mayor of Gurupi signed a contract of operation with Infraero. Previously the airport was operated by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolic Compounds In Wine
The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers ( proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids. Origin of the phenolic compounds The natural phenols are not evenly distributed within the fruit. Phenolic acids are largely present in the pulp, anthocyanins and stilbenoids in the skin, and other phenols (catechins, proanthocyanidins and flavonols) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)


