|
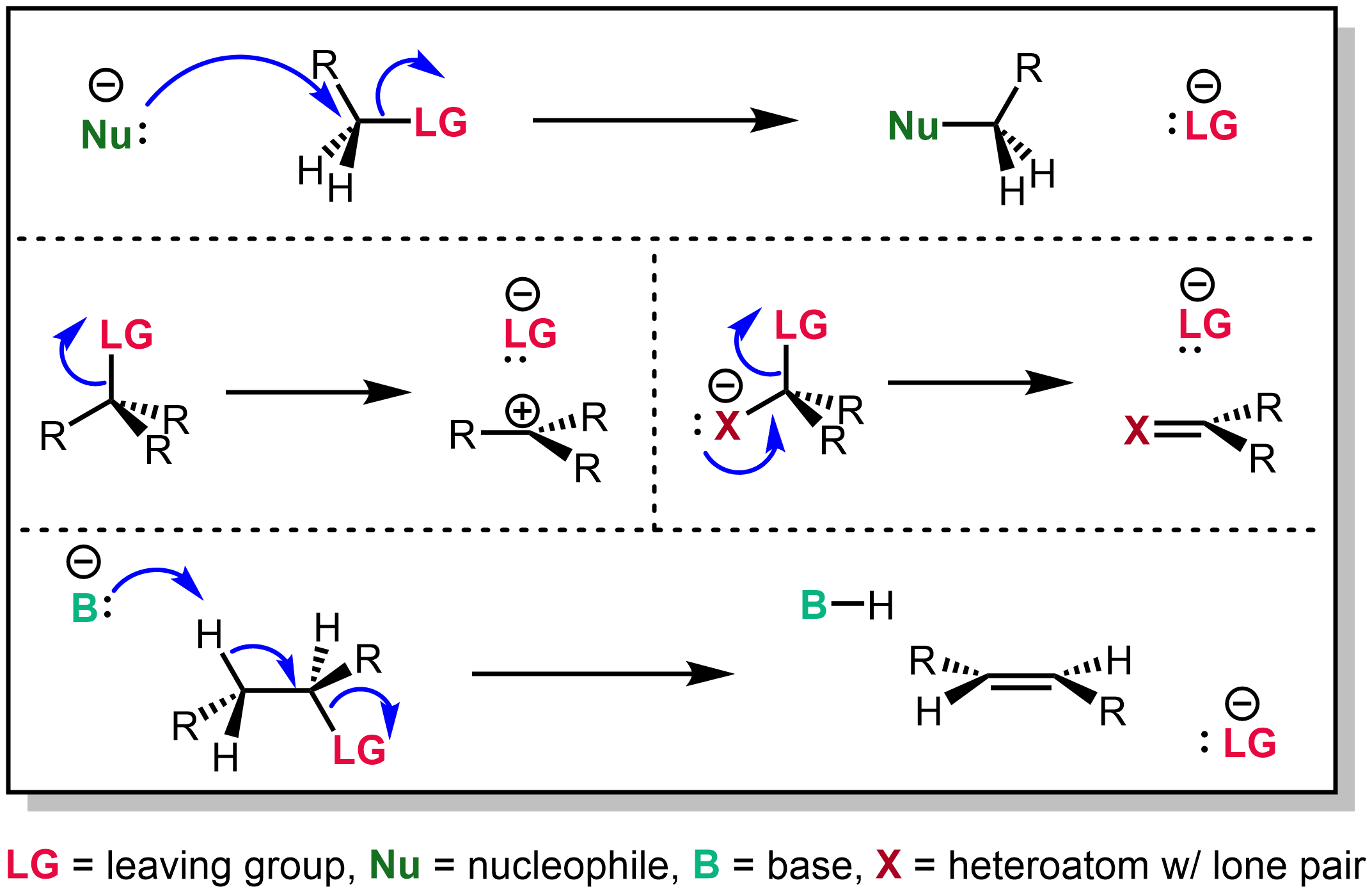
Geminal Halide Hydrolysis
Geminal halide hydrolysis is an organic reaction. The reactants are geminal dihalides with a water molecule or a hydroxide ion. The reaction yields ketones from secondary halides or aldehydes from primary halides. Reaction mechanism The first part of the reaction mechanism consists of an ordinary nucleophilic aliphatic substitution to produce a ''gem''- halohydrin: : RCH(Cl)2 + KOH \longrightarrow RCH(OH)Cl + KCl The remaining halide is a good leaving group and this enables the newly created hydroxy group to convert into a carbonyl group by expelling the halide: :RCH(OH)Cl \longrightarrowRearrangement gives R-CHO + HCl Variations Other functional groups can undergo similar hydrolysis reactions. For instance, geminal trihalides (e.g. benzotrichloride) can be partially hydrolyzed to acyl halides (e.g. benzoyl chloride) in a similar way. Further hydrolysis yields carboxylic acids. See also *Stephen aldehyde synthesis Stephen aldehyde synthesis, a named reaction i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organic Photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group (side group) has a different definition. Conventions The placeholder R is often used as a generic placeholder for alkyl (saturated hydrocarbon) group side chains in chemical structure diagrams. To indicate other non-carbon groups in structure diagrams, X, Y, or Z are often used. History The ''R'' symbol was introduced by 19th-century French chemist Charles Frédéric Gerhardt, who advocated its adoption on the grounds that it would be widely recognizable and intelligible given its correspondence in multiple European languages to the initial letter of "root" or "residue": ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoyl Chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins. Preparation Benzoyl chloride is produced from benzotrichloride using either water or benzoic acid: :C6H5CCl3 + H2O -> C6H5COCl + 2 HCl :C6H5CCl3 + C6H5CO2H -> 2 C6H5COCl + HCl As with other acyl chlorides, it can be generated from the parent acid and standard chlorinating agents such as phosphorus pentachloride, thionyl chloride, and oxalyl chloride. It was first prepared by treatment of benzaldehyde with chlorine. An early method for production of benzoyl chloride involved chlorination of benzyl alcohol. Reactions It reacts with water to produce hydrochloric acid and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Halide
In organic chemistry, an acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen). If the acid is a carboxylic acid (), the compound contains a functional group, which consists of a carbonyl group () singly bonded to a halogen atom. The general formula for such an acyl halide can be written RCOX, where R may be, for example, an alkyl group, CO is the carbonyl group, and X represents the halide, such as chloride. Acyl chlorides are the most commonly encountered acyl halides, but acetyl iodide is the one produced (transiently) on the largest scale. Billions of kilograms are generated annually in the production of acetic acid. Preparation Aliphatic acyl halides On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: :(CH3CO)2O + HCl -> CH3COCl + CH3CO2H Common syntheses of acyl chlorides al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzotrichloride
Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless (to yellowish), viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents. History Benzotrichloride is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and hence its use is subject to a list of reporting requirements by companies or institutions which synthesize, store or use it in large quantities. In 2018, EU member states have approved a European Commission proposal to restrict the use of carcinogenic, mutagenic and reprotoxic (CMR) substances in clothing, textiles and footwear. In 2015, the Commission published a preli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol, 3-chloropropane-1,2-diol). The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers. Halohydrins may be categorized as chlorohydrins, bromohydrins, fluorohydrins or iodohydrins depending on the halogen present. Synthesis From alkenes Halohydrins are usually prepared by treatment of an alkene with a halogen, in the presence of water. The reaction is a form of electrophilic addition, similar to the halogen addition reaction and proceeds with anti addition, leaving the newly added X and OH groups in a trans configuration. The chemical equation for the conversion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geminal
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to the same carbon atom, as in methanediol. Also the shortened prefix ''gem'' may be applied to a chemical name to denote this relationship, as in a ''gem''-dibromide for "geminal dibromide". The concept is important in many branches of chemistry, including synthesis and spectroscopy, because functional groups attached to the same atom often behave differently from when they are separated. Geminal diols, for example, are easily converted to ketones or aldehydes with loss of water.Peter Taylor (2002)''Mechanism and synthesis'' Book 10 of ''Molecular world''. Open University, Royal Society of Chemistry; . 368 pages The related term vicinal refers to the relationship between two functional groups that are attached to adjacent atoms. The rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |