|
Gas Mask
A gas mask is a mask used to protect the wearer from inhaling airborne pollutants and toxic gases. The mask forms a sealed cover over the nose and mouth, but may also cover the eyes and other vulnerable soft tissues of the face. Most gas masks are also respirators, though the word ''gas mask'' is often used to refer to military equipment (such as a field protective mask), the scope used in this article. The gas mask only protects the user from digesting, inhaling, and contact through the eyes (many agents affect through eye contact). Most combined gas mask filters will last around 8 hours in a biological or chemical situation. Filters against specific chemical agents can last up to 20 hours. Airborne toxic materials may be gaseous (for example, chlorine or mustard gas), or particulates (such as biological agents). Many filters provide protection from both types. The first gas masks mostly used circular lenses made of glass, mica or cellulose acetate to allow vision. Glass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A World War I British Gas Hood C
A, or a, is the first Letter (alphabet), letter and the first vowel of the Latin alphabet, Latin alphabet, used in the English alphabet, modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is English alphabet#Letter names, ''a'' (pronounced ), plural English alphabet#Letter names, ''aes''. It is similar in shape to the Greek alphabet#History, Ancient Greek letter alpha, from which it derives. The Letter case, uppercase version consists of the two slanting sides of a triangle, crossed in the middle by a horizontal bar. The lowercase version can be written in two forms: the double-storey a and single-storey ɑ. The latter is commonly used in handwriting and fonts based on it, especially fonts intended to be read by children, and is also found in italic type. In English grammar, "English articles, a", and its variant "English articles#Indefinite article, an", are Article (grammar)#Indefinite article, indefinite arti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects. Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other planets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyanide ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is ''stinkdamp''. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. The British English spelling of this compound is hydrogen sulphide, a spelling no longer recommended by the Royal Society of Chemistry or the International Union of Pure and Applied Chemistry. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or it or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexavalent Chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the salt sodium dichromate. Hexavalent chromium is key to all materials made from chromium. Approximately of hexavalent chromium were produced in 1985. Additional hexavalent chromium compounds include chromium trioxide and various salts of chromate and dichromate, among others. Hexavalent chromium is used in textile dyes, wood preservation, anti-corrosion products, chromate conversion coatings, and a variety of niche uses. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anticorrosive agents to paints, primers, and other surface coatings; and chromic acid electroplated onto metal parts to provide a decorative or protective coating. Hexavalent chromium can be fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone Cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those products owing to preventive measures. However, it does occur in many other safety-critical items such as fuel lines and rubber seals, such as gaskets and O-rings, where ozone attack is considered unlikely. Only a trace amount of the gas is needed to initiate cracking, and so these items can also succumb to the problem. Susceptible elastomers Tiny traces of ozone in the air will attack double bonds in rubber chains, with natural rubber, polybutadiene, styrene-butadiene rubber and nitrile rubber being most sensitive to degradation. Every repeat unit in the first three materials has a double bond, so every unit can be degraded by ozone. Nitrile rubber is a copolymer of butadiene and acrylonitrile units, but the proportion of acrylonitrile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riebeckite
Riebeckite is a sodium-rich member of the amphibole group of silicate minerals, chemical formula Na2(Fe2+3Fe3+2)Si8O22(OH)2. It forms a solid solution series with magnesioriebeckite. It crystallizes in the monoclinic system, usually as long prismatic crystals showing a diamond-shaped cross section, but also in fibrous, bladed, acicular, columnar, and radiating forms. Its Mohs hardness is 5.0–6.0, and its specific gravity is 3.0–3.4. Cleavage is perfect, two directions in the shape of a diamond; fracture is uneven, splintery. It is often translucent to nearly opaque. Name and discovery Riebeckite was first described in 1888 for an occurrence on Socotra Island, Aden Governorate, Yemen, and named for German explorer Emil Riebeck (1853–1885). The mineral is also known as crocidolite. Occurrence Riebeckite typically forms dark-blue elongated to fibrous crystals in highly alkali granites, syenites, rarely in felsic volcanics, granite pegmatites and schist. It occurs in banded i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
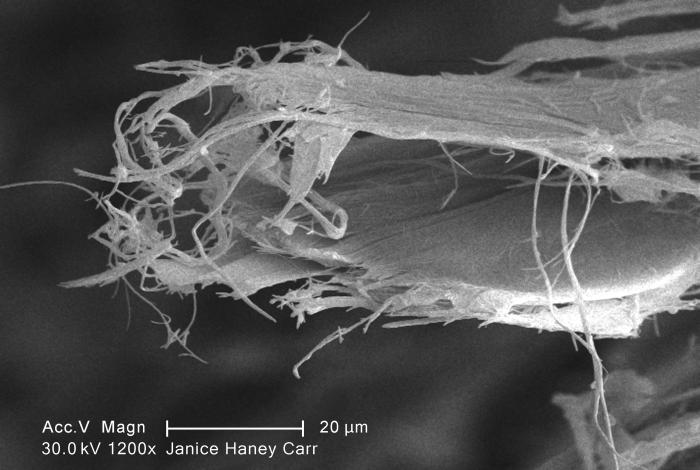
Chrysotile Asbestos
Chrysotile or white asbestos is the most commonly encountered form of asbestos, accounting for approximately 95% of the asbestos in the United StatesOccupational Safety and Health Administration, United States Department of Labor, U.S. Department of Labor (2007)29 C.F.R. 1910.1001 Appendix J. and a similar proportion in other countries.Institut national de recherche sur la sécurité (1997).Amiante." ''Fiches toxicologiques.'' n° 167. (in French) It is a soft, fibrous silicate mineral in the serpentine subgroup of phyllosilicates; as such, it is distinct from other asbestiform minerals in the amphibole group. Its idealized chemical formula is Magnesium, Mg(Silicon, SiOxygen, O)(Hydroxide, OH). The material has physical properties which make it desirable for inclusion in building materials, but poses serious health risks when dispersed into air and inhaled. Polytypes Three polytypes of chrysotile are known. These are very difficult to distinguish in hand specimens, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




-oxid.jpg)
.jpg)