|
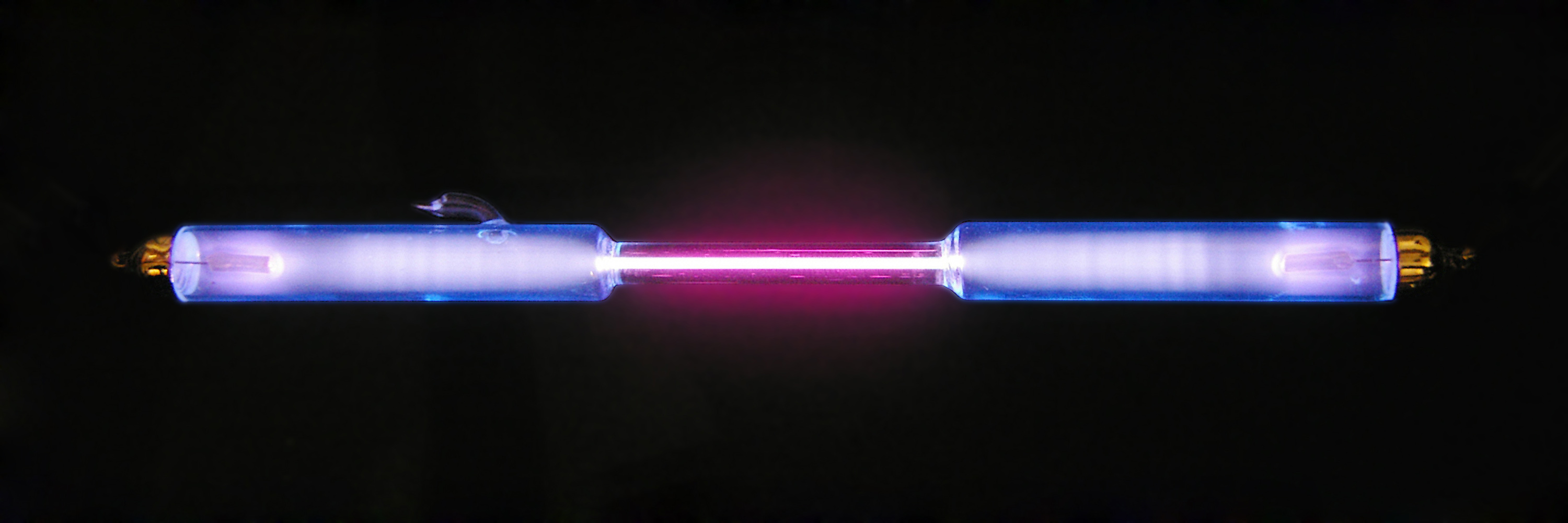
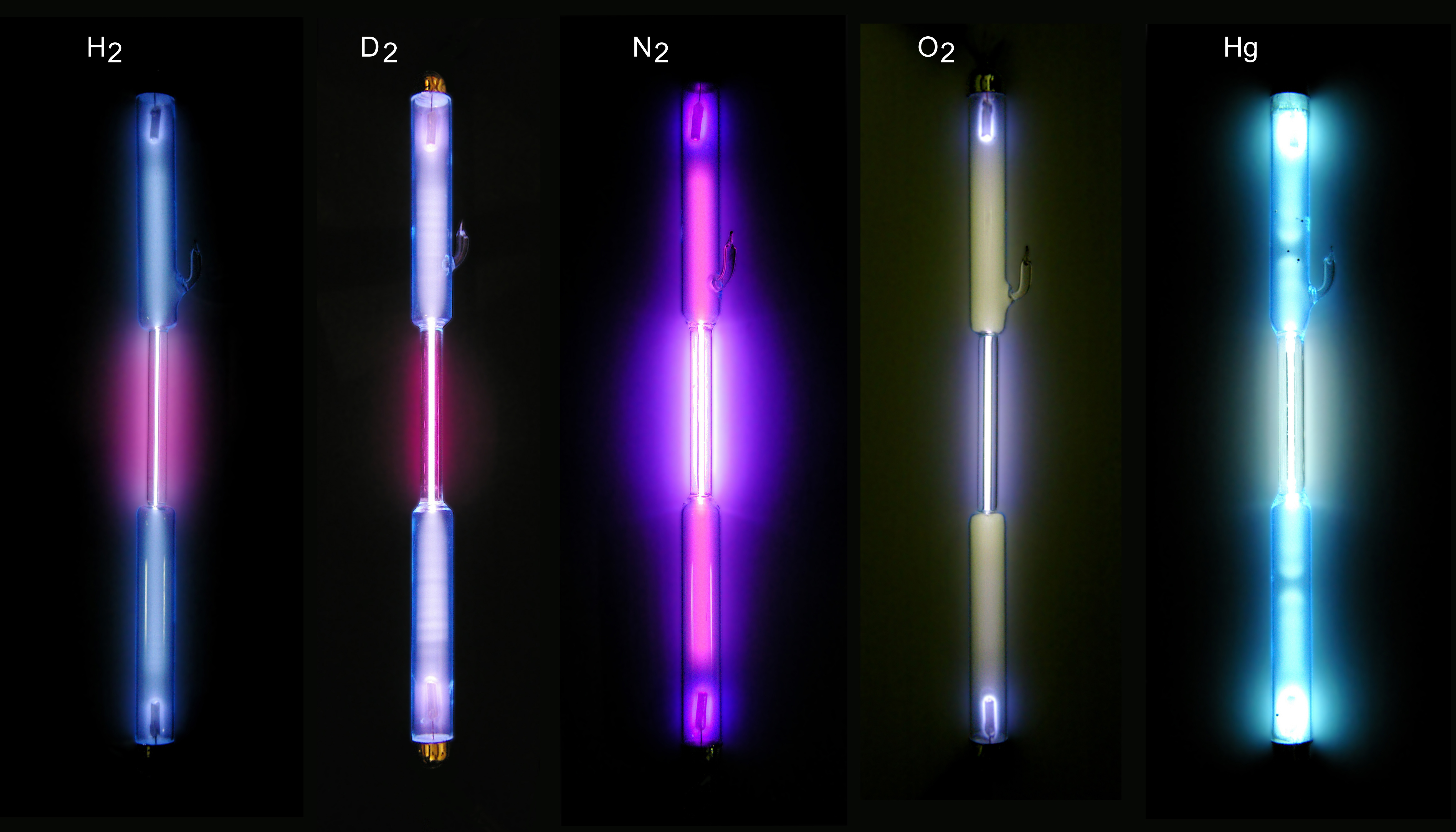
Gas-filled Tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage sufficient to cause electrical conduction by the underlying phenomena of the Townsend discharge. A gas-discharge lamp is an electric light using a gas-filled tube; these include fluorescent lamps, metal-halide lamps, sodium-vapor lamps, and neon lights. Specialized gas-filled tubes such as krytrons, thyratrons, and ignitrons are used as switching devices in electric devices. The voltage required to initiate and sustain discharge is dependent on the pressure and composition of the fill gas and geometry of the tube. Although the envelope is typically glass, power tubes often use ceramics, and military tubes often use glass-lined metal. Both hot cathode and cold cath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyratron
A thyratron is a type of gas-filled tube used as a high-power electrical switch and controlled rectifier. Thyratrons can handle much greater currents than similar hard-vacuum tubes. Electron multiplication occurs when the gas becomes ionized, producing a phenomenon known as Townsend discharge. Gases used include mercury vapor, xenon, neon, and (in special high-voltage applications or applications requiring very short switching times) hydrogen. Unlike a vacuum tube (valve), a thyratron cannot be used to amplify signals linearly. In the 1920s, thyratrons were derived from early vacuum tubes such as the UV-200, which contained a small amount of argon gas to increase its sensitivity as a radio signal detector, and the German LRS relay tube, which also contained argon gas. Gas rectifiers, which predated vacuum tubes, such as the argon-filled General Electric " Tungar bulb" and the Cooper-Hewitt mercury-pool rectifier, also provided an influence. Irving Langmuir and G. S. Meikle of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crossatron
In electronics, a crossatron is a high-power pulsed modulator device, a cold cathode gas-filled tube that combines the best features of thyratrons, vacuum tubes, and power semiconductor switches. This switch is capable of operating with voltages in excess of 100 kilovolts by the use of deuterium gas fill to increase the Paschen breakdown voltage, axial molybdenum cathode corrugations to provide a higher current capability, and a Paschen shield that is formed from molybdenum. The terminal curvature of the Paschen shield and of the adjacent portion of the anode are selected to establish a voltage stress at the curved Paschen shield surface within the approximate range of 90–150 kV/cm in response to a 100 kV differential. The cold cathode gives the crossatron an advantage of achievable lifetime and reliability in comparison to a hydrogen-filled thyratron. It features instant start and rugged operation while enduring high temperatures, high radiation, electromagnetic pulse, and r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Generator
Neutron generators are neutron source devices which contain compact linear particle accelerators and that produce neutrons by fusing isotopes of hydrogen together. The fusion reactions take place in these devices by accelerating either deuterium, tritium, or a mixture of these two isotopes into a metal hydride target which also contains deuterium, tritium or a mixture of these isotopes. Fusion of deuterium atoms (D + D) results in the formation of a helium-3 ion and a neutron with a kinetic energy of approximately 2.5 MeV. Fusion of a deuterium and a tritium atom (D + T) results in the formation of a helium-4 ion and a neutron with a kinetic energy of approximately 14.1 MeV. Neutron generators have applications in medicine, security, and materials analysis. The basic concept was first developed by Ernest Rutherford's team in the Cavendish Laboratory in the early 1930s. Using a linear accelerator driven by a Cockcroft–Walton generator, Mark Oliphant led an experiment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet Spectroscopy
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into contact. For huma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dekatron
In electronics, a Dekatron (or Decatron, or generically three-phase gas counting tube or glow-transfer counting tube or cold cathode tube) is a gas-filled decade counting tube. Dekatrons were used in computers, calculators, and other counting-related products during the 1950s and 1960s. "Dekatron," now a generic trademark, was the brand name used by Ericsson Telephones Limited (ETL), of Beeston, Nottingham (not to be confused with the Swedish TelefonAB Ericsson of Stockholm). The device was invented by John Reginald Acton, with the patent assigned to Ericsson. US2651004A The dekatron was useful for computing, calculating, and frequency-dividing purposes because one complete revolution of the neon dot in a dekatron means 10 pulses on the guide electrode(s), and a signal can be derived from one of the ten cathodes in a dekat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cold Cathode
A cold cathode is a cathode that is not electrically heated by a filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" if it emits more electrons than can be supplied by thermionic emission alone. It is used in gas-discharge lamps, such as neon lamps, discharge tubes, and some types of vacuum tube. The other type of cathode is a hot cathode, which is heated by electric current passing through a filament. A cold cathode does not necessarily operate at a low temperature: it is often heated to its operating temperature by other methods, such as the current passing from the cathode into the gas. Cold-cathode devices A cold-cathode vacuum tube does not rely on external heating of an electrode to provide thermionic emission of electrons. Early cold-cathode devices included the Geissler tube and Plucker tube, and early cathode-ray tubes. Study of the phenomena in these devi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
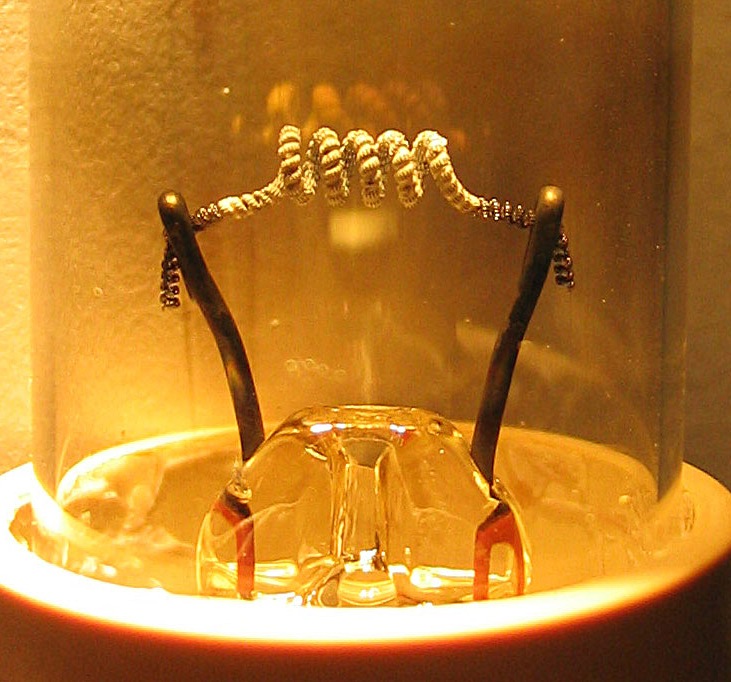
Hot Cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. The heating element is usually an electrical filament heated by a separate electric current passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathodes rely on field electron emission or secondary electron emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a ''directly heated cathode'', the filament is the cathode and emits the electrons. In an ''indirectly heated cathode'', the filament or ''heater'' heats a separate metal cathode electrode which emits the electrons. From the 1920s to the 1960s, a wide variety of electronic devices used hot-cathode vacuum tubes. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |