|
Fullerene Ligands
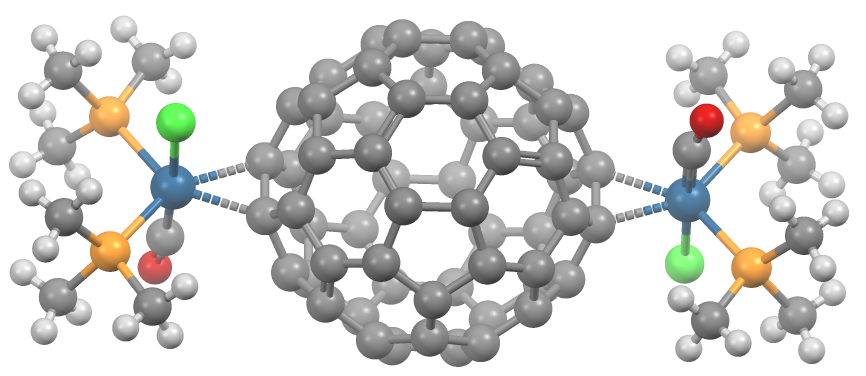
A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60. One year after it was prepared in milligram quantities in 1990, C60 was shown to function as a ligand in the complex h3Psub>2Pt(η2-C60). Since this report, a variety of transition metals and binding modes were demonstrated. Most transition metal fullerene complex are derived from C60, although other fullerenes also coordinate to metals as seen with C70Rh(H)(CO)(PPh3)2. Binding modes As ligands, fullerenes behave similarly to electron-deficient alkenes such as tetracyanoethylene. Thus, their complexes are a subset of metal-alkene complexes. They almost always coordinate in a dihapto fashion and prefer electron-rich metal centers.Spessard, p. 162 This binding occurs on the junction of two 6-membered rings. Hexahapto and pentahapto bonding is rarely observed.Spessard, p. 165 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football ("soccer"). Nested closed fullerenes have been named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fullerenes
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football ("soccer"). Nested closed fullerenes have been named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors. Buckminsterfullerene is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The compound was discovered in 1985 and has received intense study, although few real world applications have been found. Occurrence Buckminsterfullerene is the most common naturally occurring fullerene. Small quantities of it can be found in soot. It also exists in space. Neutral C60 has been observed in planetary nebulae and several types of star. The ionised form, C60+, has been identified in the interstellar medium, where it is the cause of several absorption features known as diffuse interstellar bands in the near-infrared. History Theoretical predictions of buckyball molecules appeared in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron-deficient
Electron deficiency (and electron-deficient) is jargon that is used in two contexts: species that violate the octet rule because they have too few valence electrons and species that happen to follow the octet rule but have electron-acceptor properties, forming donor-acceptor charge-transfer salts. Octet rule violations left, 144px Traditionally, "electron-deficiency" is used as a general descriptor for boron hydrides and other molecules which do not have enough valence electrons to form localized (2-centre 2-electron) bonds joining all atoms. For example, diborane (B2H6) would require a minimum of 7 localized bonds with 14 electrons to join all 8 atoms, but there are only 12 valence electrons. A similar situation exists in trimethylaluminium. The electron deficiency in such compounds is similar to metallic bonding. Electron-acceptor molecules Alternatively, electron-deficiency describes molecules or ions that function as electron acceptors. Such electron-deficient species obey ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetracyanoethylene
Tetracyanoethylene (TCNE) is organic compound with the formula . It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons. Synthesis and reactions TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper. Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties. In the presence of base, TCNE reacts with malononitrile to give salts of pentacyanopropenide: :C2(CN)2 + CH2(CN)2 -> NC)2C-C(CN)-C(CN)2 + CN- + 2H+ Redox chemistry TCNE is an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems (conjugated) to the central double bond, gives rise to an electrophilic alkene. TCNE is reduced at -0.27 V vs ferrocene/ferrocenium: :C2(CN)4 + e- -> 2(CN)4 Because of its ability to accept an electron, TCNE has been used to prepare numerou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal-alkene Complex
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. Mono- and dialkenes are often used as ligands in stable complexes. Monoalkenes The simplest monoalkene is ethene. Many complexes of ethene are known, including Zeise's salt (see figure), Rh2Cl2(C2H4)4, Cp*2Ti(C2H4), and the homoleptic Ni(C2H4)3. Substituted monoalkene include the cyclic cyclooctene, as found in chlorobis(cyclooctene)rhodium dimer. Alkenes with electron-withdrawing groups commonly bind strongly to low-valent metals. Examples of such ligands are TCNE, tetrafluoroethylene, maleic anhydride, and esters of fumaric acid. These acceptors form adducts with many zero-valent metals. Dienes, trienes, polyenes, keto-alkenes, and other complicated alkene ligands Butadiene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Orgel, and Rich described the structure of the "sandwich complex" ferrocene by X-ray crystallography where an iron atom is ''"sandwiched"'' between two parallel cyclopen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)



