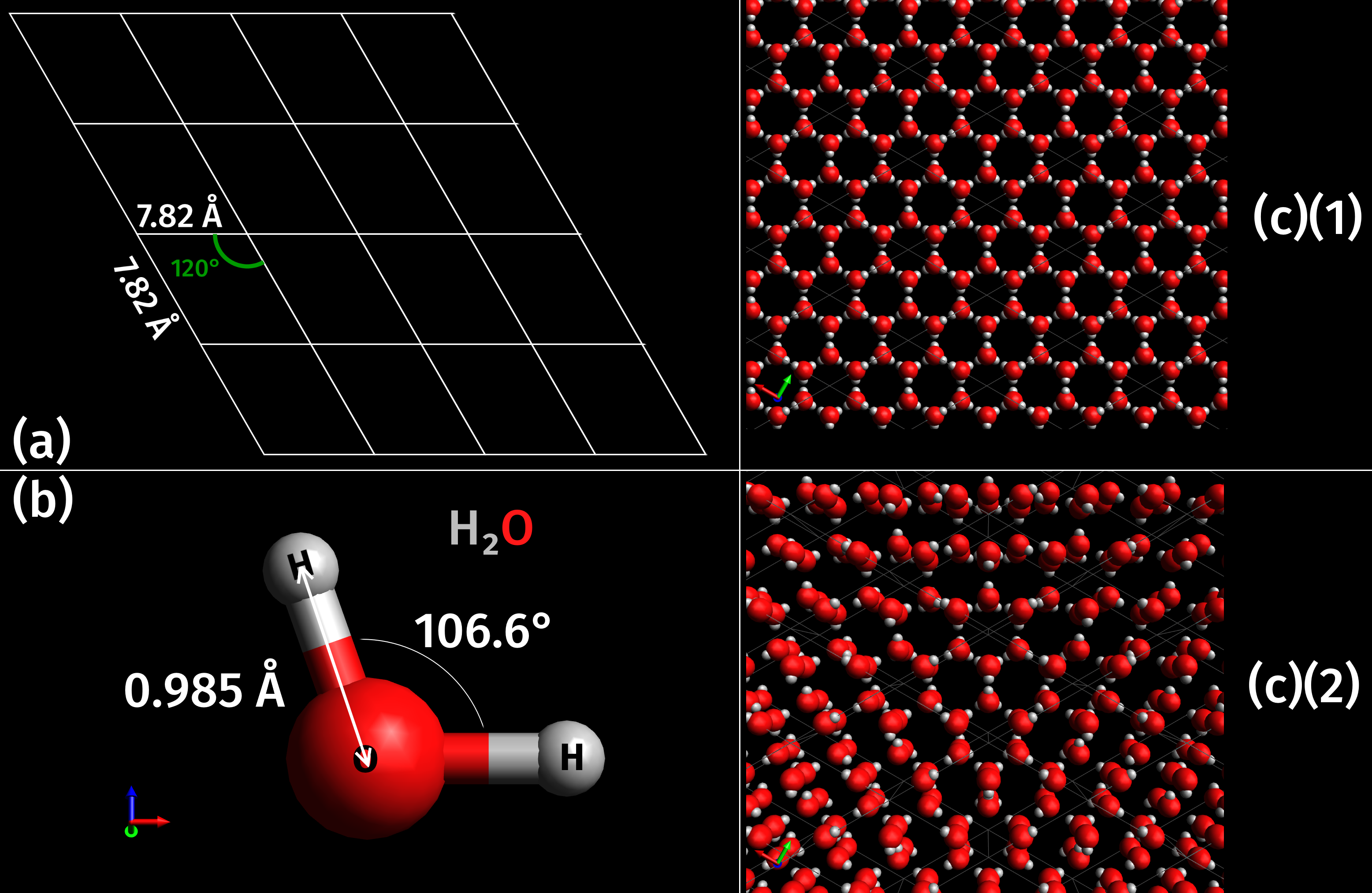
|
Freeze Distillation
Fractional freezing is a process used in process engineering and chemistry to Separation process, separate substances with different melting points. It can be done by partial melting of a solid, for example in zone melting, zone refining of silicon or metals, or by partial crystallization of a liquid, as in freeze distillation, also called normal freezing or progressive freezing. The initial sample is thus fractionation, fractionated (separation process, separated into fraction (chemistry), fractions). Partial crystallization can also be achieved by adding a dilute solvent to the mixture, and cooling and concentrating the mixture by evaporating the solvent, a process called solution crystallization. Fractional freezing is generally used to produce ultra-pure solids, or to concentrate heat-sensitive liquids. Freeze distillation Freeze distillation is a misnomer, because it is not distillation but rather a process of enriching a solution by partially freezing it and removing fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Applejack (beverage)
Applejack is a strong alcoholic drink produced from apples. Popular in the American colonial era, the drink's prevalence declined in the 19th and 20th centuries amid competition from other spirits.Michael Foley, ''Drinking with the Saints: The Sinner's Guide to a Holy Happy Hour'' (2015, ): Perhaps the most interesting option is applejack, the first distilled liquor native to North America and a great favorite among the colonists. owusually a blend of apple brandy and neutral spirits that retains the flavor of the apples /ref> Applejack is used in several cocktails, including the Jack Rose. It is a type of fruit brandy. History Apple brandy was first produced in colonial New Jersey in 1698 by William Laird, a Scottish immigrant who settled in Monmouth County.Karen Tina HarrisonJersey Lightning ''New Jersey Monthly'', July 13, 2009. The drink was once known as Jersey Lightning. Laird's great-grandson, Robert Laird, who served in the Continental Army, incorporated Laird's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Wine Grapes
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. In the Solar System, ice is abundant and occurs naturally from as close to the Sun as Mercury to as far away as the Oort cloud objects. Beyond the Solar System, it occurs as interstellar ice. It is abundant on Earth's surfaceparticularly in the polar regions and above the snow lineand, as a common form of precipitation and deposition, plays a key role in Earth's water cycle and climate. It falls as snowflakes and hail or occurs as frost, icicles or ice spikes and aggregates from snow as glaciers and ice sheets. Ice exhibits at least eighteen phases ( packing geometries), depending on temperature and pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form depending on its hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polynyas
A polynya () is an area of open water surrounded by sea ice. It is now used as a geographical term for an area of unfrozen seawater within otherwise contiguous pack ice or fast ice. It is a loanword from the Russian полынья (), which refers to a natural ice hole and was adopted in the 19th century by polar explorers to describe navigable portions of the sea. There are two main types of polynyas: coastal polynyas, which can be found year-round near the Antarctic and Arctic coasts and are mainly created by strong winds pushing the ice away from the coast, and mid-sea or open-ocean polynyas, which may be found more sporadically in the middle of ice pack in certain locations, especially around Antarctica. These locations are generally preconditioned by certain oceanic dynamics. One of the most famous mid-sea polynyas is the Weddell Polynya, also known as the Maud Rise Polynya, which occurs in the Lazarev Sea over the Maud Rise seamount. It was first spotted in September 1973 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brine Rejection
Brine rejection is a process that occurs when salty water freezes. The salts do not fit in the crystal structure of water ice, so the salt is expelled. Since the oceans are salty, this process is important in nature. Salt rejected by the forming sea ice drains into the surrounding seawater, creating saltier, denser brine. The denser brine sinks, influencing ocean circulation. Formation As water reaches the temperature where it begins to crystallize and form ice, salt ions are rejected from the lattices within the ice and either forced out into the surrounding water, or trapped among the ice crystals in pockets called brine cells. Generally, sea ice has a salinity ranging from 0 psu at the surface to 4 psu at the base. The faster that this freezing process occurs, the more brine cells are left in the ice. Once the ice reaches a critical thickness, roughly 15 cm, the concentration of salt ions in the liquid around the ice begins to increase, as leftover brine is rejected from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is . How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in Soil salinity control, soil desalination, which is an issue for agriculture. Saline water, Saltwater (especially Seawater, sea water) is desalinated to produce water suitable for Drinking water, human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources. Due to its energy consumption, desalinating sea water is generally more costly than fresh water from surface water or groundwater, Reclaimed water, water recycling and water conservation. However, these alternatives are not always available and depletion of reserve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentrate
A concentrate is a form of Chemical substance, substance that has had the majority of its base component (in the case of a liquid: the solvent) removed. Typically, this will be the removal of water from a Solution (chemistry), solution or suspension (chemistry), suspension, such as the removal of water from fruit juice. One benefit of producing a concentrate is that of a reduction in weight and volume for transportation, as the concentrate can be reconstituted at the time of usage by the addition of the solvent. Soft drink concentrates The process of concentrating orange juice was patented in 1939. It was originally developed to provide World War II troops with a reliable source of vitamin C. Most Soft drink, sodas and soft drinks are produced as highly concentrated syrups and later diluted with carbonated water directly before consumption or bottling. Such concentrated syrups are sometimes retailed to the end-consumer because of their relatively low price and considerable weig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fruit Juice
Juice is a drink made from the extraction or pressing of the natural liquid contained in fruit and vegetables. It can also refer to liquids that are flavored with concentrate or other biological food sources, such as meat or seafood, such as clam juice. Juice is commonly consumed as a beverage or used as an ingredient or flavoring in foods or other beverages, as for smoothies. Juice emerged as a popular beverage choice after the development of pasteurization methods enabled its preservation without using fermentation (which is used in wine production). The largest fruit juice consumers are New Zealand (nearly a cup, or 8 ounces, each day) and Colombia (more than three quarters of a cup each day). Fruit juice consumption on average increases with a country's income level. Etymology The word "juice" comes from Old French in about 1300; it developed from the Old French words "''jus, juis, jouis''", which mean "liquid obtained by boiling herbs". The "Old French ''jus'' "juice, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
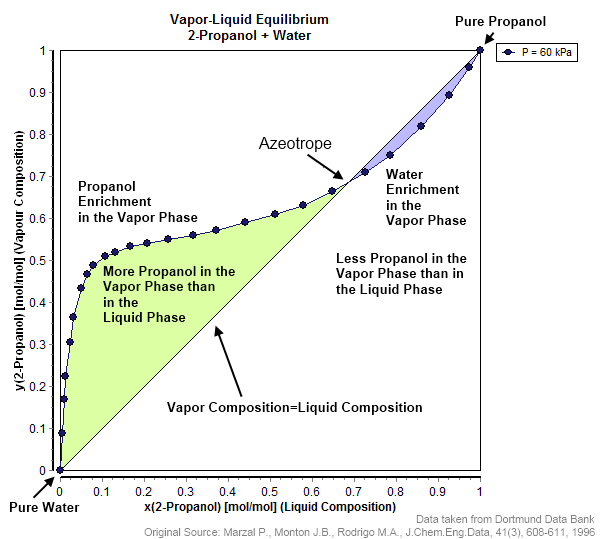
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) ''constant boiling point mixtures''. Some azeotropic mixtures of pairs of compounds are known, and many azeotropes of three or more compounds are also known. In such a case it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. There are two types of azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A Solution (chemistry), solution that shows greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |