|
Formylation Reaction
A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate esters (''O''-CH=O). A reagent that delivers the formyl group is called a formylating agent. A particularly important formylation process is hydroformylation which converts alkenes to the homologated aldehyde. The conversion of benzene to benzaldehyde is the basis of the Gattermann–Koch reaction: Aromatic formylation Formylation reactions are a form of electrophilic aromatic substitution and therefore work best when the aromatic starting materials are electron-rich. Phenols are very commonly encountered as they can be readily deprotonated to form phenoxides which are excellent nucleophiles, other electron rich substrates such as mesitylene, pyrrole, or fused aromatic rings can also be expected to react. Benzene will react under aggressive condi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde2
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Alde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds. Preparation Mesitylene is prepared by transalkylation of xylene over solid acid catalyst:Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke “Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. . :2 C6H4(CH3)2 ⇌ C6H3( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
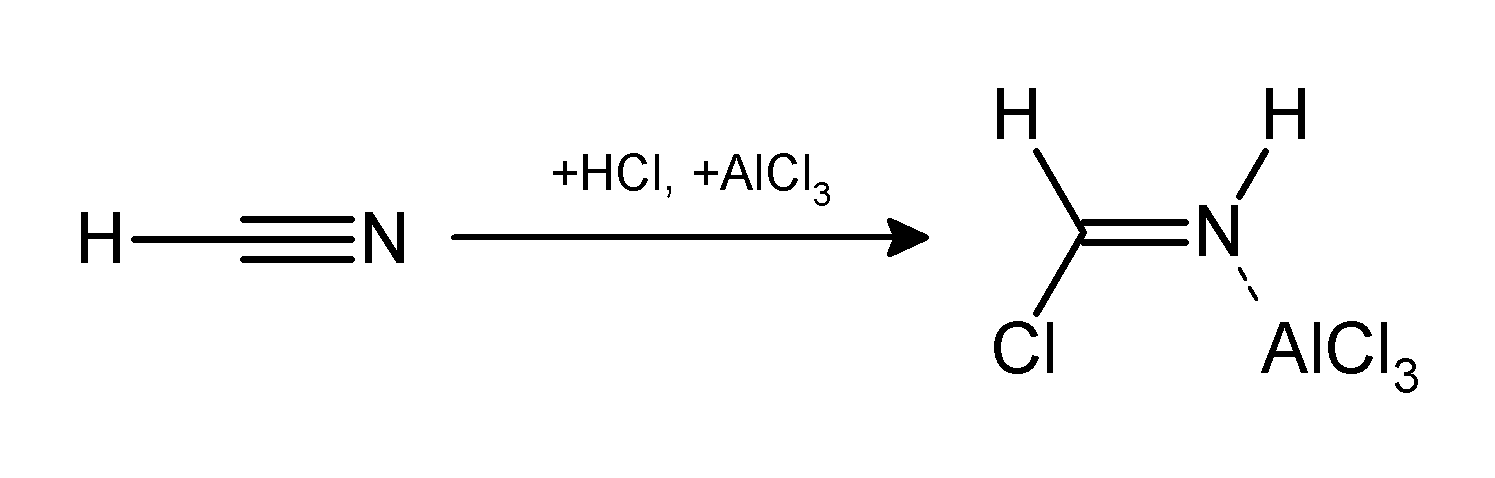
Gattermann-Koch Reaction
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann–Koch reaction, nam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gattermann Reaction
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann–Koch reaction, nam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Formaldehyde Resin
Phenol formaldehyde resins (PF) or phenolic resins (also infrequently called phenoplasts) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial synthetic resins (plastics). They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesives. They were at one time the primary material used for the production of circuit boards but have been largely replaced with epoxy resins and fiberglass cloth, as with fire-resistant FR-4 circuit board materials. There are two main production methods. One reacts phenol and formaldehyde directly to produce a thermosetting network polymer, while the other restricts the formaldehyde to produce a prepolymer known as novolac which can be moulded and then cured with the addition of more formaldehyde and heat.A. Gardziella, L.A. Pilato, A. Knop, Phenolic Resins: Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Casiraghi Formylation
Casiraghi is an Italian surname. Notable people with the surname include: * Andrea Casiraghi (born 1984), 2nd in line to the Monegasque throne after his mother, Caroline, Princess of Hanover * Charlotte Casiraghi (born 1986), 4th in line to the Monegasque throne after her mother, Caroline, Princess of Hanover * Cinzia Casiraghi, an Italian physicist who won the Sofia Kovalevskaya Award * Daniele Casiraghi, Italian footballer * Sister Leonarda Angela Casiraghi, recipient of the Padma Shri Awards * Pierluigi Casiraghi, (born 1969), Italian football player * Pierre Casiraghi (born 1987), 3rd in line to the Monegasque throne after his mother, Caroline, Princess of Hanover * Rosagnese Casiraghi, mayor of Missaglia Missaglia ( Brianzöö: ) is a ''comune'' (municipality) in the Province of Lecco in the Italian Lombardy region, located at the centre of the area known as the Meratese. As of 31 December 2004, it had a population of 7,805. The comune, which ... * Stefano Casiraghi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraformaldehyde
Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Paraformaldehyde is a poly-acetal. Synthesis Paraformaldehyde forms slowly in aqueous formaldehyde solutions as a white precipitate, especially if stored in the cold. Formalin actually contains very little monomeric formaldehyde; most of it forms short chains of polyformaldehyde. A small amount of methanol is often added as a stabilizer to limit the extent of polymerization. Reactions Paraformaldehyde can be depolymerized to formaldehyde gas by dry heating and to formaldehyde solution by water in the presence of a base, an acid or heat. The high purity formaldehyde solutions obtained in this way are used as a fixative for microscopy and histology. The resulting formaldehyde gas from dry heating paraformaldehyde is flammable. Uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kolbe–Schmitt Reaction
The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by heating sodium phenoxide (the sodium salt of phenol) with carbon dioxide under pressure (100 atm, 125 °C), then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid (the precursor to aspirin). 500px, center, The Kolbe–Schmitt reaction By using potassium hydroxide, 4-hydroxybenzoic acid is accessible, an important precursor for the versatile paraben class of biocides used e.g. in personal care products. The methodology is also used in the industrial synthesis of 3-hydroxy-2-naphthoic acid; the regiochemistry of the carboxylation in this case is sensitive to temperature.. Reaction mechanism The Kolbe–Schmitt reaction proceeds via the nucleophilic addition of a phenoxide, classically sodium phenoxide (NaOC6H5), to carbon dioxide to give the salicylate. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
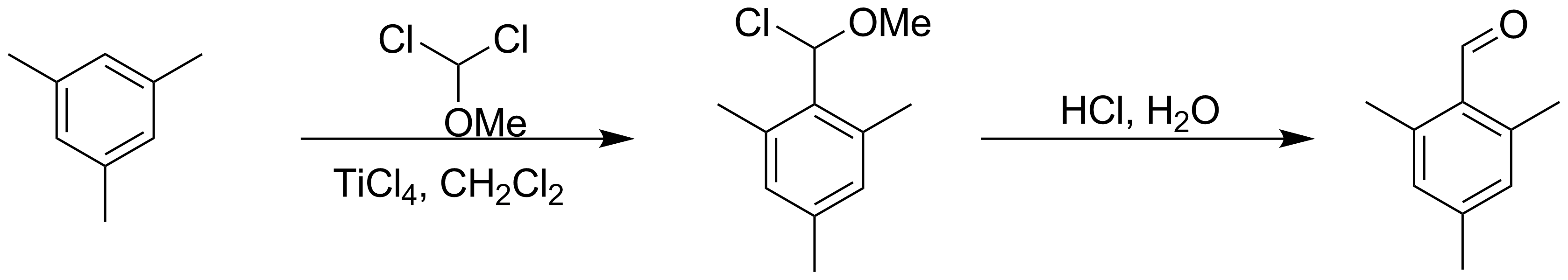
Rieche Formylation
Rieche formylation is a type of formylation reaction. The substrates are electron rich aromatic compounds, such as mesitylene or phenols, with dichloromethyl methyl ether acting as the formyl source. The catalyst is titanium tetrachloride and the workup is acidic. The reaction is named after Alfred Rieche Alfred Rieche (28 April 1902 – 6 November 2001) was a German chemist A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study t ... who discovered it in 1960. See also Reimer–Tiemann reaction. References {{Reflist Organic reactions Formylation reactions Carbon-carbon bond forming reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duff Reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. It is named after James Cooper Duff, who was a chemist at the College of Technology, Birmingham, around 1920–1950. The electrophilic species in this electrophilic aromatic substitution reaction is the iminium ion CH2+NR2. The initial reaction product is an iminium which is hydrolyzed to the aldehyde. See mechanism below. The reaction requires strongly electron donating substituents on the aromatic ring such as in a phenol. Formylation occurs '' ortho'' to the electron donating substituent preferentially, unless the ''ortho'' positions are blocked, in which case the formylation occurs at the ''para'' position. Examples are the synthesis of 3,5-di-''tert''-butylsalicylaldehyde: and the synthesis of syringaldehyde: If both ''ortho'' positions are vacant then a diformylation is possible, as in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vilsmeier–Haack Reaction
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted amide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5). The reaction is named after Anton Vilsmeier and Albrecht Haack. For example, benzanilide and dimethylaniline react with phosphorus oxychloride to produce an unsymmetrical diaryl ketone. Similarly, anthracene is formylated at the 9-position. The reaction of anthracene with ''N''-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde: : Reaction mechanism The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion (2), also called the Vilsmeier reagent. The initial product is an iminium ion (4b), which is hydrolyzed to the corresponding ketone or aldehyde during workup. : See also * Formylation reaction A formylation reaction in organic chemistry refers to organic reactions in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-hydroxybenzaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula (C7 H6 O2) C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important. Production Salicylaldehyde is prepared from phenol and chloroform by heating with sodium hydroxide or potassium hydroxide in a Reimer–Tiemann reaction: : Alternatively, it is produced by condensation of phenol or its derivatives with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde. Salicylaldehydes in general may be prepared by other ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base. Natural occurr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
molybdenum_tricarbonyl.png)