|
Fluorosilicic Acid
Hexafluorosilicic acid is an inorganic compound with the chemical formula . Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless. Hexafluorosilicic acid is produced naturally on a large scale in volcanoes.Palache, C., Berman, H., and Frondel, C. (1951) Dana’s System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc. John Wiley and Sons, Inc., New York, 7th edition.Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (1997) Handbook of Mineralogy, Volume III: Halides, Hydroxides, Oxides. Mineral Data Publishing, Tucson.link to bararite It is manufactured as a coproduct in the production of phosphate fertilizers. The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing. Salts der ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Hexafluorosilicate
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid.Wiberg, E., Wiberg, N., and Holleman, A. F. (2001) ''Inorganic chemistry''. Academic Press, San Diego. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite. Structure Ammonium fluorosilicate has three major polymorphs: α-(NH4)2 iF6form is cubic (space group Fm3m, No. 225) and corresponds to the mineral cryptohalite. The β form is trigonal (scalenohedral) and occurs in nature as mineral bararite. A third (γ) form was discovered in 2001 and identified with the hexagonal 6mm symmetry. In all three configurations, the iF6sup>2− octahedra are arranged in layers. In the α form, these layers are perpendicular to 11directions. In the β- and γ- forms, the layers are perpendicular to the c-axis. ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. We ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hexafluorosilicate
Sodium fluorosilicate is a compound with the chemical formula Na2 iF6 Natural occurrence Sodium hexafluorosilicate occurs naturally as the rare mineral malladrite found within some volcanic fumaroles. Manufacturing Sodium fluorosilicate is made by neutralizing fluorosilicic acid with sodium chloride or sodium sulfate. :H2 iF6+ 2 NaCl → Na2 iF6+ 2 HCl Possible application It is used in some countries as additives for water fluoridation, opal glass raw material, ore refining, or other fluoride chemical (like sodium fluoride, magnesium silicofluoride, cryolite, aluminum fluoride) production. See also * Fluorosilicic acid * Ammonium fluorosilicate Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid.Wiberg, E., Wiberg, N., and Holleman, A. ... References {{silicon compounds Sodium compounds Hexafluorosilicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hexafluorosilicate
Sodium fluorosilicate is a compound with the chemical formula Na2 iF6 Natural occurrence Sodium hexafluorosilicate occurs naturally as the rare mineral malladrite found within some volcanic fumaroles. Manufacturing Sodium fluorosilicate is made by neutralizing fluorosilicic acid with sodium chloride or sodium sulfate. :H2 iF6+ 2 NaCl → Na2 iF6+ 2 HCl Possible application It is used in some countries as additives for water fluoridation, opal glass raw material, ore refining, or other fluoride chemical (like sodium fluoride, magnesium silicofluoride, cryolite, aluminum fluoride) production. See also * Fluorosilicic acid * Ammonium fluorosilicate Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid.Wiberg, E., Wiberg, N., and Holleman, A. ... References {{silicon compounds Sodium compounds Hexafluorosilicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth Metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure. Structurally, they (together with helium) have in common an outer s-orbital which is full; that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with charge +2, and an oxidation state of +2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the decay chain of uranium and thorium and not as a primordial element. There have been experiments, all unsuccessful, to try to synthesize element 120, the next potential member of the group. Characteristics Chemical As with other groups, the members of this family show patterns in their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
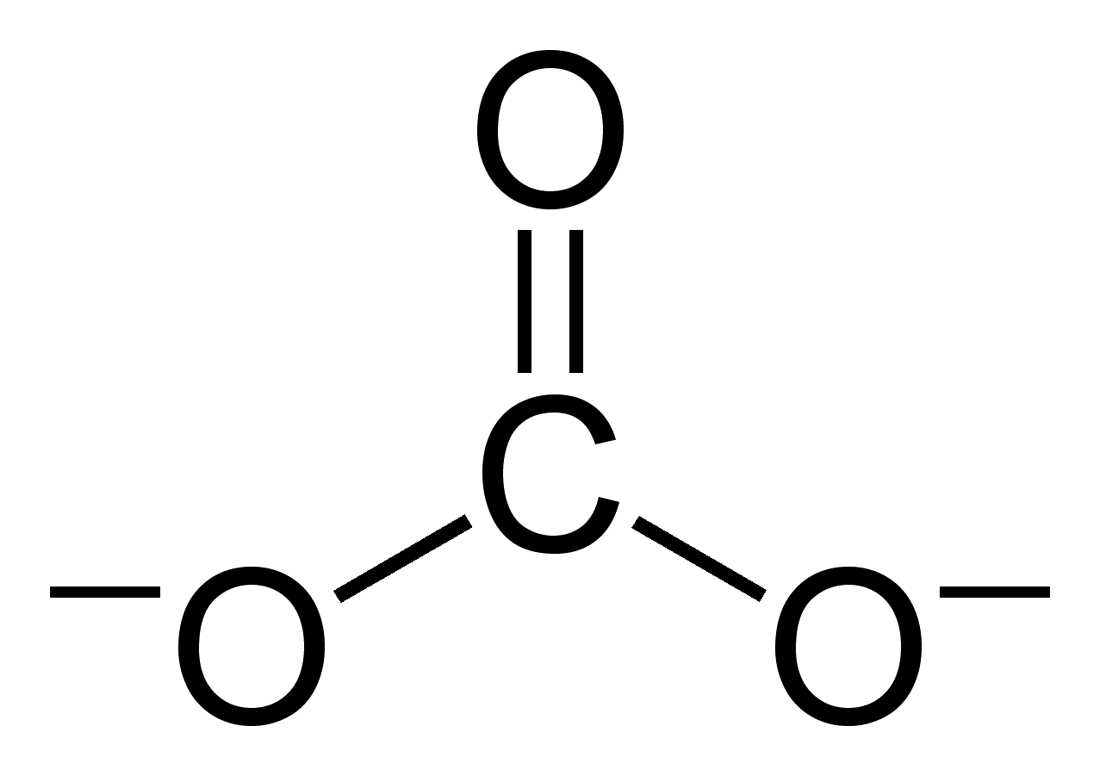
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is a natural par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an atomic orbital, s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised homology (chemistry), homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material polytetrafluoroethylene, PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to Etching (microfabrication), etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Polytetrafluoroethylene, Teflon, fluoropolymers, fluorocarbons, and refrigeration, refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2SiF6%2C_ICSDcode_40388full.png)






_0468.jpg)