|
Fluorimetry
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores. Devices that measure fluorescence are called fluorometers. Theory Molecules have various states referred to as energy levels. Fluorescence spectroscopy is primarily concerned with electronic and vibrational states. Generally, the species being examined has a ground electronic state (a low energy state) of interest, and an excited electronic state of higher energy. Within each of these elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorimeter
A fluorometer, fluorimeter or fluormeter is a device used to measure parameters of visible spectrum fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion. Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology. Components and Design Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorometer
A fluorometer, fluorimeter or fluormeter is a device used to measure parameters of visible spectrum fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion. Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology. Components and Design Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
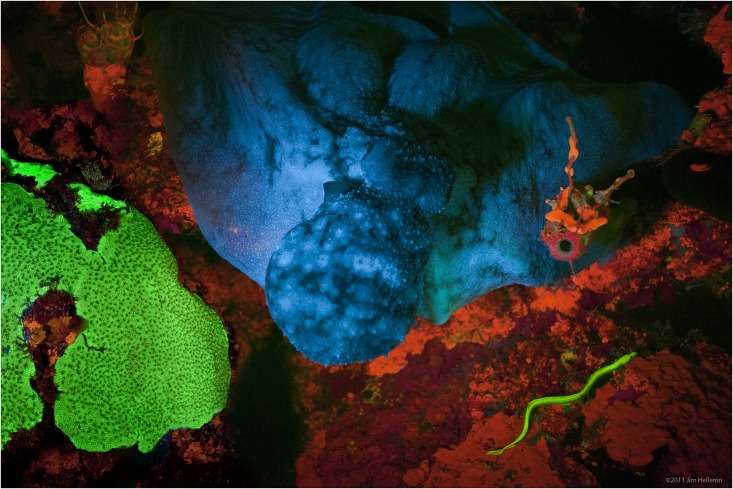
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSA 2
PSA, PsA, Psa, or psa may refer to: Biology and medicine * Posterior spinal artery * Primary systemic amyloidosis, a disease caused by the accumulation of abnormal proteins * Prostate-specific antigen, an enzyme used as a blood tracer for prostate cancer * Psoriatic arthritis (PsA), an inflammatory disease * ''Pseudomonas aeruginosa'', a species of bacterium * ''Pseudomonas syringae'' pv. ''actinidiae'', a pathovar of a bacterium that attacks kiwifruit Chemistry * Polar surface area, the surface sum over all polar atoms of a molecule * Pressure swing adsorption, a technology for separating, or purifying, gases Computing * Professional services automation, software for automating project and billing management for professional service firms * PSA Certified, Platform Security Architecture, a security certification for the Internet of Things * Plesk Server Administrator, a commercial web hosting automation program Contracts, legislation, and government * Passenger Vessel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury-vapor Lamp
A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from the ultraviolet radiation the light produces, and a convenient mounting for the fused quartz arc tube. Mercury vapor lamps are more energy efficient than incandescent lamps with luminous efficacies of 35 to 55 lumens/watt. Their other advantages are a long bulb lifetime in the range of 24,000 hours and a high intensity, clear white light output. For these reasons, they are used for large area overhead lighting, such as in factories, warehouses, and sports arenas as well as for streetlights. Clear mercury lamps produce a greenish light due to mercury's combination of spectral lines. This is not flatt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collimated
A collimated beam of light or other electromagnetic radiation has parallel rays, and therefore will spread minimally as it propagates. A perfectly collimated light beam, with no divergence, would not disperse with distance. However, diffraction prevents the creation of any such beam. Light can be approximately collimated by a number of processes, for instance by means of a collimator. Perfectly collimated light is sometimes said to be ''focused at infinity''. Thus, as the distance from a point source increases, the spherical wavefronts become flatter and closer to plane waves, which are perfectly collimated. Other forms of electromagnetic radiation can also be collimated. In radiology, X-rays are collimated to reduce the volume of the patient's tissue that is irradiated, and to remove stray photons that reduce the quality of the x-ray image ("film fog"). In scintigraphy, a gamma ray collimator is used in front of a detector to allow only photons perpendicular to the surface to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stray Light
Stray light is light in an optical system, which was not intended in the design. The light may be from the intended source, but follow paths other than intended, or it may be from a source other than the intended source. This light will often set a working limit on the dynamic range of the system; it limits the signal-to-noise ratio or contrast ratio, by limiting how dark the system can be. Ocular straylight is stray light in the human eye. Optical systems Monochromatic light Optical measuring instruments that work with monochromatic light, such as spectrophotometers, define stray light as light in the system at wavelengths (colors) other than the one intended. The stray light level is one of the most critical specifications of an instrument. For instance, intense, narrow absorption bands can easily appear to have a peak absorption less than the true absorption of the sample because the ability of the instrument to measure light transmission through the sample is limited by the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', ''number concentration'', and ''volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Etymology The term concentration comes from the word concentrate, from the French , from con– + center, meaning “to put at the center”. Qualitative description Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" for solutions of relatively low concentration and "concentrated" for solutions of relatively high concentration. To concentrate a solution, one must add more solute (for example, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensity (physics)
In physics, the intensity or flux of radiant energy is the Power (physics), power transferred per unit area, where the area is measured on the plane perpendicular to the direction of propagation of the energy. In the SI system, it has units watts per square metre (W/m2), or kilogram, kg⋅second, s−3 in SI base unit, base units. Intensity is used most frequently with waves such as acoustic waves (sound) or electromagnetic waves such as light or radio waves, in which case the time averaging, ''average'' power transfer over one Period (physics), period of the wave is used. ''Intensity'' can be applied to other circumstances where energy is transferred. For example, one could calculate the intensity of the kinetic energy carried by drops of water from a garden sprinkler. The word "intensity" as used here is not synonymous with "wikt:strength, strength", "wikt:amplitude, amplitude", "wikt:magnitude, magnitude", or "wikt:level, level", as it sometimes is in colloquial speech. Intensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |