|
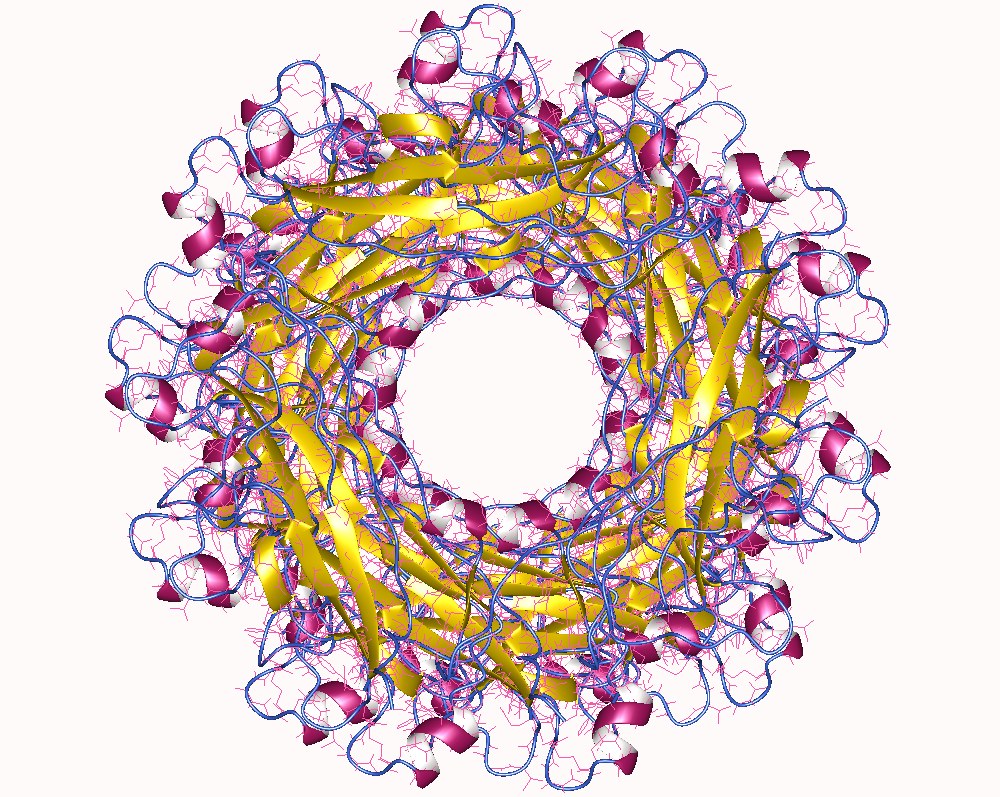
Fimbrial Usher Protein
The fimbrial usher protein is involved in biogenesis of the pilus in Gram-negative bacteria Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall .... The biogenesis of some fimbriae (or pili) requires a two-component assembly and transport system which is composed of a periplasmic chaperone and a pore-forming outer membrane protein which has been termed a molecular 'usher'; this is the chaperone-usher pathway. The usher protein has a molecular weight ranging from 86 to 100 kDa and is composed of a membrane-spanning 24-stranded beta barrel domain, reminiscent of porins, and of four periplasmic soluble domains: an N-terminal one of about 120 residues (NTD), a 'middle' domain of about 80 residues located as a soluble insertion within the beta barrel region of the sequence (plug domain) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pilus
A pilus (Latin for 'hair'; plural: ''pili'') is a hair-like appendage found on the surface of many bacteria and archaea. The terms ''pilus'' and '' fimbria'' (Latin for 'fringe'; plural: ''fimbriae'') can be used interchangeably, although some researchers reserve the term ''pilus'' for the appendage required for bacterial conjugation. All conjugative pili are primarily composed of pilin – fibrous proteins, which are oligomeric. Dozens of these structures can exist on the bacterial and archaeal surface. Some bacteria, viruses or bacteriophages attach to receptors on pili at the start of their reproductive cycle. Pili are antigenic. They are also fragile and constantly replaced, sometimes with pili of different composition, resulting in altered antigenicity. Specific host responses to old pili structures are not effective on the new structure. Recombination genes of pili code for variable (V) and constant (C) regions of the pili (similar to immunoglobulin diversity). As the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative Bacteria
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism ''Escherichia coli'', as well as many pathogenic bacteria, such as ''Pseudomonas aeruginosa'', '' Chlamydia trachomatis'', and ''Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a complex lipopol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone-usher Pathway
Chaperone-usher fimbriae (CU) are linear, unbranching, outer-membrane pili secreted by gram-negative bacteria through the chaperone-usher system rather than through type IV secretion or extracellular nucleation systems. These fimbriae are built up out of modular pilus subunits, which are transported into the periplasm in a Sec dependent manner. Chaperone-usher secreted fimbriae are important pathogenicity factors facilitating host colonisation, localisation and biofilm formation in clinically important species such as uropathogenic ''Escherichia coli'' and ''Pseudomonas aeruginosa''. Structure Overall All chaperone/usher systems are found within gene clusters consisting of at least an usher, a chaperone and one or more fimbriae subunits. Overall the system includes periplasmic chaperones, periplasmic and extracellular pilus subunits, dimeric usher outer membrane pore and associated Sec machinery. The Pilus subunits polymerise via a non-covalent interaction to form the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porin (protein)
Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria (mycolic acid-containing actinomycetes), the outer membrane of mitochondria, and the outer chloroplast membrane (outer plastid membrane). Structure Porins are composed of beta sheets (β sheets) made up of beta strands (β strands) which are linked together by beta turns on the cytoplasmic side and long loops of amino acids on the other. The β strands lie in an antiparallel fashion and form a cylindrical tube, called a beta barrel (β barrel). The amino acid composition of the porin β strands are unique in that polar and nonpolar residues alternate along them. This means that the nonpolar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |