|
Fast Breeder
A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. Breeder reactors achieve this because their neutron economy is high enough to create more fissile fuel than they use, by irradiation of a fertile material, such as uranium-238 or thorium-232, that is loaded into the reactor along with fissile fuel. Breeders were at first found attractive because they made more complete use of uranium fuel than light water reactors, but interest declined after the 1960s as more uranium reserves were found,Helmreich, J.E. ''Gathering Rare Ores: The Diplomacy of Uranium Acquisition, 1943–1954'', Princeton UP, 1986: ch. 10 and new methods of uranium enrichment reduced fuel costs. Fuel efficiency and types of nuclear waste Breeder reactors could, in principle, extract almost all of the energy contained in uranium or thorium, decreasing fuel requirements by a factor of 100 compared to widely used once-through light water reactors, which extract less th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transuranic
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. With the exception of neptunium and plutonium (which have been found in trace amounts in nature), all do not occur naturally on Earth and are synthetic. Overview Of the elements with atomic numbers 1 to 92, most can be found in nature, having stable isotopes (such as hydrogen) or very long-lived radioisotopes (such as uranium), or existing as common decay products of the decay of uranium and thorium (such as radon). The exceptions are elements 43, 61, 85, and 87; all four occur in nature, but only in very minor branches of the uranium and thorium decay chains, and thus all save element 87 were first discovered by synthesis in the laboratory rather than in nature (and even element 87 was discovered from purified samples of its parent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Hindu
''The Hindu'' is an Indian English-language daily newspaper owned by The Hindu Group, headquartered in Chennai, Tamil Nadu. It began as a weekly in 1878 and became a daily in 1889. It is one of the Indian newspapers of record and the second most circulated English-language newspaper in India, after '' The Times of India''. , ''The Hindu'' is published from 21 locations across 11 states of India. ''The Hindu'' has been a family-owned newspaper since 1905, when it was purchased by S. Kasturi Ranga Iyengar from the original founders. It is now jointly owned by Iyengar's descendants, referred to as the "Kasturi family", who serve as the directors of the holding company. The current chairperson of the group is Malini Parthasarathy, a great-granddaughter of Iyengar. Except for a period of about two years, when S. Varadarajan held the editorship of the newspaper, the editorial positions of the paper were always held by members of the family or held under their direction. Histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doubling Time
The doubling time is the time it takes for a population to double in size/value. It is applied to population growth, inflation, resource extraction, consumption of goods, compound interest, the volume of malignant tumours, and many other things that tend to grow over time. When the relative growth rate (not the absolute growth rate) is constant, the quantity undergoes exponential growth and has a constant doubling time or period, which can be calculated directly from the growth rate. This time can be calculated by dividing the natural logarithm of 2 by the exponent of growth, or approximated by dividing 70 by the percentage growth rate (more roughly but roundly, dividing 72; see the rule of 72 for details and derivations of this formula). The doubling time is a characteristic unit (a natural unit of scale) for the exponential growth equation, and its converse for exponential decay is the half-life. For example, given Canada's net population growth of 0.9% in the year 2006, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PHWR
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water ( deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for pressurized water reactor. While heavy water is very expensive to isolate from ordinary water (often referred to as ''light water'' in contrast to ''heavy water''), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all current ope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling through a small sphere of radius R in a time interval, divided by \pi R^2 (the cross section of the sphere) and by the time interval. The usual unit is cm−2s−1 (neutrons per centimeter squared per second). The neutron fluence is defined as the neutron flux integrated over a certain time period, so its usual unit is cm−2 (neutrons per centimeter squared). An older term used instead of cm−2 was n.v.t. (neutrons, velocity, time). Natural neutron flux Neutron flux in asymptotic giant branch stars and in supernovae is responsible for most of the natural nucleosynthesis producing elements heavier than iron. In stars there is a relatively low neutron flux on the order of 105 to 1011 cm−2 s−1, resulting in nucleosynthesis by the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Plutonium
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years, plutonium-242 with a half-life of 373,300 years, and plutonium-239 with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states; all have half-lives of less than one second. The isotopes of plutonium range in atomic weight from 228.0387 u (228Pu) to 247.074 u (247Pu). The primary decay modes before the most stable isotope, 244Pu, are spontaneous fission and alpha emission; the primary mode after is beta emission. The pri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
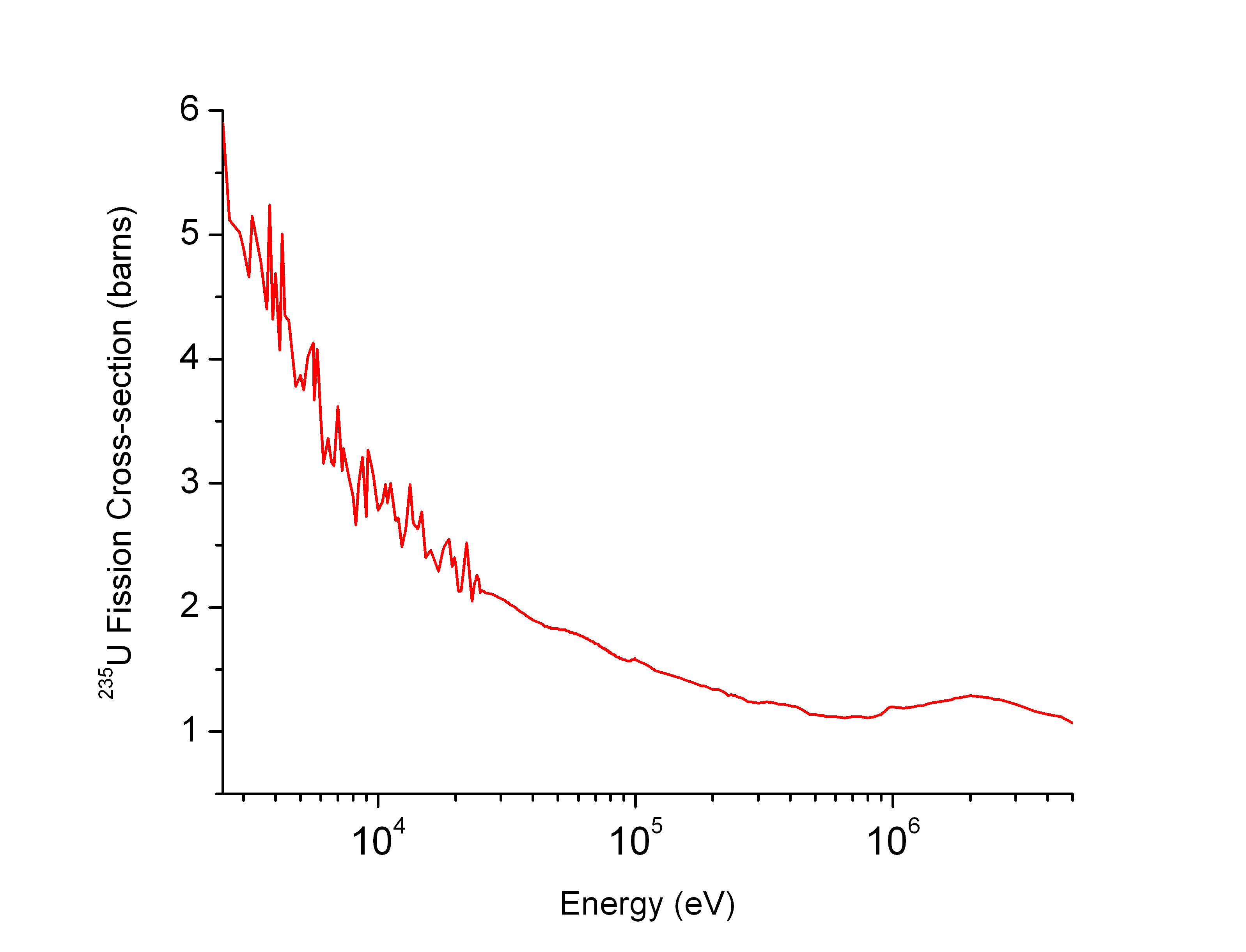
Uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Natural decay chain :\begin \ce \begin \ce \\ \ce \end \ce \\ \ce \begin \ce \\ \ce \end \ce \end Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/ mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barn (unit)
A barn (symbol: b) is a metric unit of area equal to (100 fm2). Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is also used in all fields of high-energy physics to express the cross sections of any scattering process, and is best understood as a measure of the probability of interaction between small particles. A barn is approximately the cross-sectional area of a uranium nucleus. The barn is also the unit of area used in nuclear quadrupole resonance and nuclear magnetic resonance to quantify the interaction of a nucleus with an electric field gradient. While the barn never was an SI unit, the SI standards body acknowledged it in the 8th SI Brochure (superseded in 2019) due to its use in particle physics. Etymology During Manhattan Project research on the atomic bomb during World War II, American physicists at Purdue University needed a secretive name for a unit with which to quantify the cross-secti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spent Nuclear Fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and depending on its point along the nuclear fuel cycle, it may have considerably different isotopic constituents. The term "fuel" is slightly confusing, as it implies a combustion of some type, which does not occur in a nuclear power plant. Nevertheless, this term is generally accepted. Nature of spent fuel Nanomaterial properties In the oxide fuel, intense temperature gradients exist that cause fission products to migrate. The zirconium tends to move to the centre of the fuel pellet where the temperature is highest, while the lower-boiling fission products move to the edge of the pellet. The pellet is likely to contain many small bubble-like pores that form during use; the fission product xenon migrates to these voids. Some of this xeno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minor Actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium (element 93), americium (element 95), curium (element 96), berkelium (element 97), californium (element 98), einsteinium (element 99), and fermium (element 100). The most important isotopes of these elements in spent nuclear fuel are neptunium-237, americium-241, americium-243, curium-242 through -248, and californium-249 through -252. Plutonium and the minor actinides will be responsible for the bulk of the radiotoxicity and heat generation of used nuclear fuel in the long term (300 to 20,000 years in the future). The plutonium from a power reactor tends to have a greater amount of plutonium-241 than the plutonium generated by the lower burnup operations designed to create weapons-grade plutonium. Because the reactor-grade plutonium contains so much 241Pu, the presence of americium-241 makes the pluton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous. Plutonium was first synthetically produced and isolated in late 1940 and early 1941, by a deuteron bombardment of uranium-238 in the cyclotron at the University of California, Berkeley. First, neptunium-238 ( half-life 2.1 days) was synthesized, which subsequently beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |