|
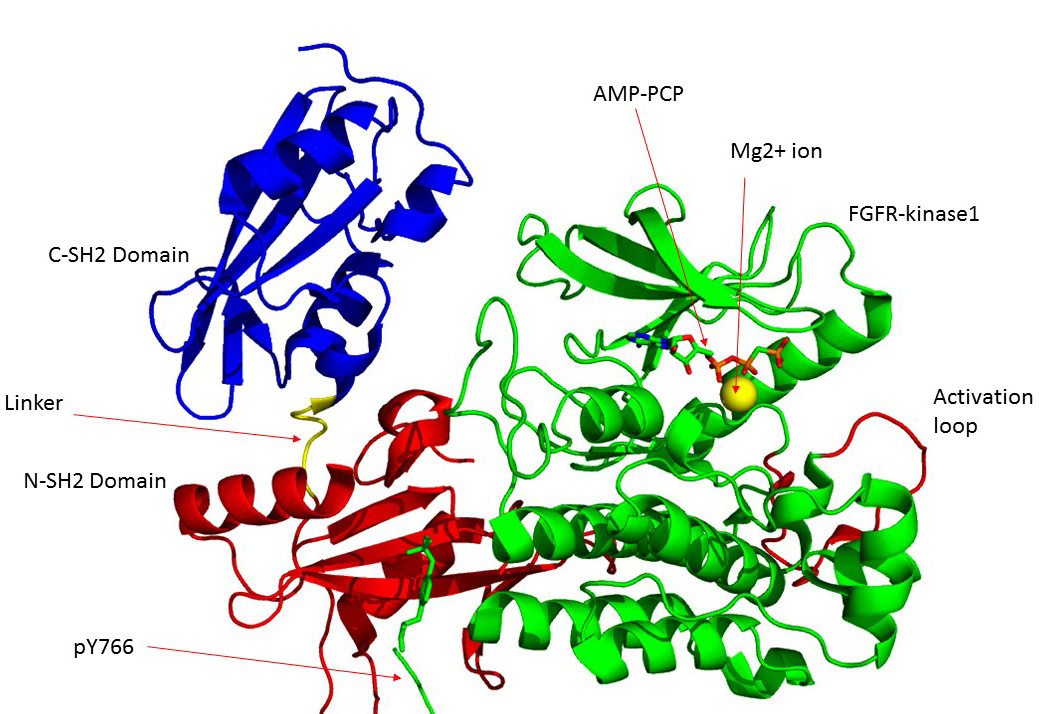
FGFR1
Fibroblast growth factor receptor 1 (FGFR1), also known as basic fibroblast growth factor receptor 1, fms-related tyrosine kinase-2 / Pfeiffer syndrome, and CD331, is a receptor tyrosine kinase whose ligands are specific members of the fibroblast growth factor family. FGFR1 has been shown to be associated with Pfeiffer syndrome, and clonal eosinophilias. Gene The ''FGFR1'' gene is located on human chromosome 8 at position p11.23 (i.e. 8p11.23), has 24 exons, and codes for a Precursor mRNA that is alternatively spliced at exons 8A or 8B thereby generating two mRNAs coding for two FGFR1 isoforms, FGFR1-IIIb (also termed FGFR1b) and FGFR1-IIIc (also termed FGFR1c), respectively. Although these two isoforms have different tissue distributions and FGF-binding affinities, FGFR1-IIIc appears responsible for most of functions of the FGFR1 gene while FGFR1-IIIb appears to have only a minor, somewhat redundant functional role. There are four other members of the ''FGFR1'' gene family: FGF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clonal Eosinophilia
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell. The clone of eosinophils bear a mutation in any one of several genes that code for proteins that regulate cell growth. The mutations cause these proteins to be continuously active and thereby to stimulate growth in an uncontrolled and continuous manner. The expanding population of eosinophils initially formed in the bone marrow may spread to the blood and then enter into and injure various tissues and organs. Clinically, clonal eosinophilia resembles various types of chronic or acute leukemias, lymphom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfeiffer Syndrome
Pfeiffer syndrome is a rare genetic disorder characterized by the premature fusion of certain bones of the skull (craniosynostosis) which affects the shape of the head and face. In addition, the syndrome includes abnormalities of the hands (such as wide and deviated thumbs) and feet (such as wide and deviated big toes). Pfeiffer syndrome is caused by mutations in the fibroblast growth factor receptors ''FGFR1'' and ''FGFR2''. The syndrome is grouped into three types, type 1 (classic Pfeiffer syndrome) being milder and caused by mutations in either gene and types 2 and 3 being more severe, often leading to death in infancy, caused by mutations in ''FGFR2''. There is no cure for the syndrome. Treatment is supportive and often involves surgery in the earliest years of life to correct skull deformities and respiratory function. Most individuals with Pfeiffer syndrome type 1 have a normal intelligence and life span, while types 2 and 3 typically result in neurodevelopmental disorde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibroblast Growth Factor
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in their function lead to a range of developmental defects. These growth factors typically act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and to heparan sulfate. Thus, some are sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and are released locally upon injury or tissue remodeling. Families In humans, 23 members of the FGF family have been identified, all of which are ''structurally'' related signaling molecules: * Members FGF1 through FGF10 all bind fibroblast growth factor receptors (FGFRs). FGF1 is also known as ''acidic fibroblast growth factor'', and FGF2 is also known a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibroblast Growth Factors
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in their function lead to a range of developmental defects. These growth factors typically act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and to heparan sulfate. Thus, some are sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and are released locally upon injury or tissue remodeling. Families In humans, 23 members of the FGF family have been identified, all of which are ''structurally'' related signaling molecules: * Members FGF1 through FGF10 all bind fibroblast growth factor receptors (FGFRs). FGF1 is also known as ''acidic fibroblast growth factor'', and FGF2 is also known as '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Phosphorylation
Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become either activated or deactivated, or otherwise modifying its function. Approximately 13000 human proteins have sites that are phosphorylated. The reverse reaction of phosphorylation is called dephosphorylation, and is catalyzed by protein phosphatases. Protein kinases and phosphatases work independently and in a balance to regulate the function of proteins. The amino acids most commonly phosphorylated are serine, threonine, tyrosine in eukaryotes, and also histidine in prokaryotes and plants (though it is now known to be common in humans). These phosphorylations play important and well-characterized roles in signaling pathways and metabolism. However, other amino acids can also be phosphory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FGFR2
Fibroblast growth factor receptor 2 (FGFR2) also known as CD332 (cluster of differentiation 332) is a protein that in humans is encoded by the ''FGFR2'' gene residing on chromosome 10. FGFR2 is a receptor for fibroblast growth factor. The protein encoded by this gene is a member of the fibroblast growth factor receptor family, where amino acid sequence is highly conserved between members and throughout evolution. FGFR family members differ from one another in their ligand affinities and tissue distribution. A full-length representative protein consists of an extracellular region, composed of three immunoglobulin domains, a single hydrophobic membrane-spanning segment and a cytoplasmic tyrosine kinase domain. The extracellular portion of the protein interacts with fibroblast growth factors, setting in motion a cascade of downstream signals, ultimately influencing mitogenesis and differentiation. This particular family member is a high-affinity receptor for acidic, basic and/or ker ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Duplication
Gene duplication (or chromosomal duplication or gene amplification) is a major mechanism through which new genetic material is generated during molecular evolution. It can be defined as any duplication of a region of DNA that contains a gene. Gene duplications can arise as products of several types of errors in DNA replication and repair machinery as well as through fortuitous capture by selfish genetic elements. Common sources of gene duplications include ectopic recombination, retrotransposition event, aneuploidy, polyploidy, and replication slippage. Mechanisms of duplication Ectopic recombination Duplications arise from an event termed unequal crossing-over that occurs during meiosis between misaligned homologous chromosomes. The chance of it happening is a function of the degree of sharing of repetitive elements between two chromosomes. The products of this recombination are a duplication at the site of the exchange and a reciprocal deletion. Ectopic recombination is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA Splicing
RNA splicing is a process in molecular biology where a newly-made precursor messenger RNA (pre-mRNA) transcript is transformed into a mature messenger RNA (mRNA). It works by removing all the introns (non-coding regions of RNA) and ''splicing'' back together exons (coding regions). For nuclear-encoded genes, splicing occurs in the nucleus either during or immediately after transcription. For those eukaryotic genes that contain introns, splicing is usually needed to create an mRNA molecule that can be translated into protein. For many eukaryotic introns, splicing occurs in a series of reactions which are catalyzed by the spliceosome, a complex of small nuclear ribonucleoproteins (snRNPs). There exist self-splicing introns, that is, ribozymes that can catalyze their own excision from their parent RNA molecule. The process of transcription, splicing and translation is called gene expression, the central dogma of molecular biology. Splicing pathways Several methods of RNA splici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
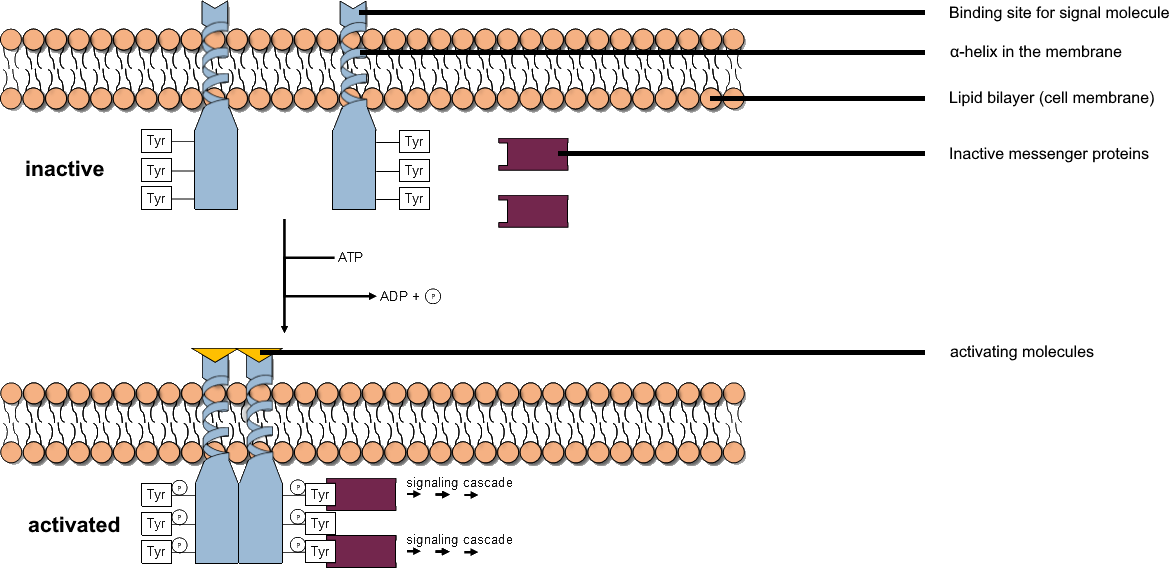
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FRS2
Fibroblast growth factor receptor substrate 2 is a protein that in humans is encoded by the ''FRS2'' gene. FRS2 is an 80 kDa membrane-anchored signal transducing adaptor protein (STAP) that links specific activated Receptor Tyrosine Kinases (RTKs) to multiple downstream signaling pathways, most notably the MAPK/ERK pathway, MAPK/ERK, PI3K/AKT/mTOR pathway, PI3K/AKT/mTOR and Phospholipase C, PLCγ pathways. It is overexpressed and amplified in several cancer types, including prostate cancer. Interactions FRS2 has been shown to Protein-protein interaction, interact with: * CBL (gene), CBL * Fibroblast growth factor receptor 1, FGFR1 * GRB2 * PRKCI * PTPN11 * SOS1 * TrkA * Anaplastic lymphoma kinase, ALK References Further reading * * * * * * * * * * * * * * * * * * {{PDB Gallery, geneid=10818 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |