|
FE-13
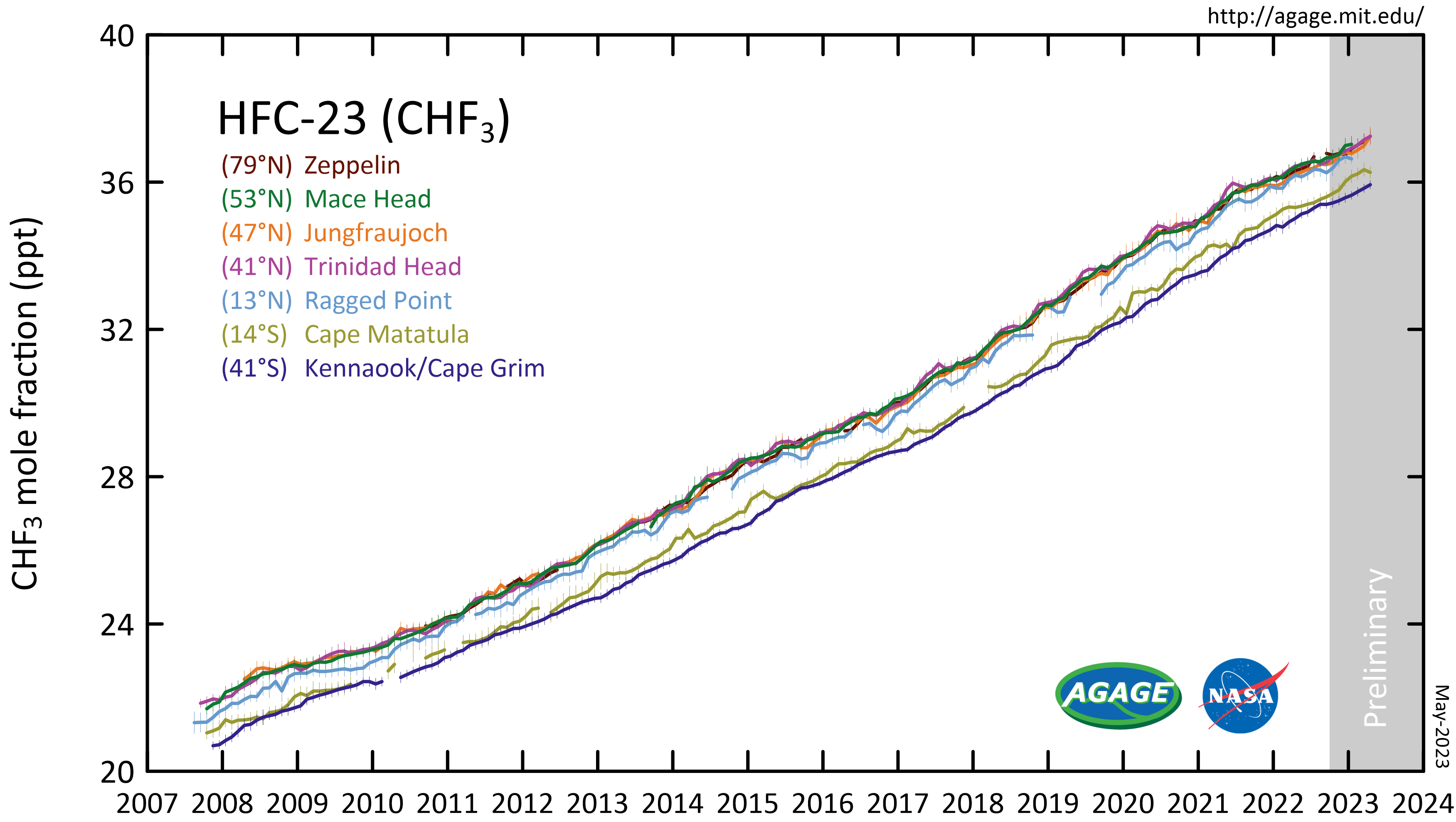
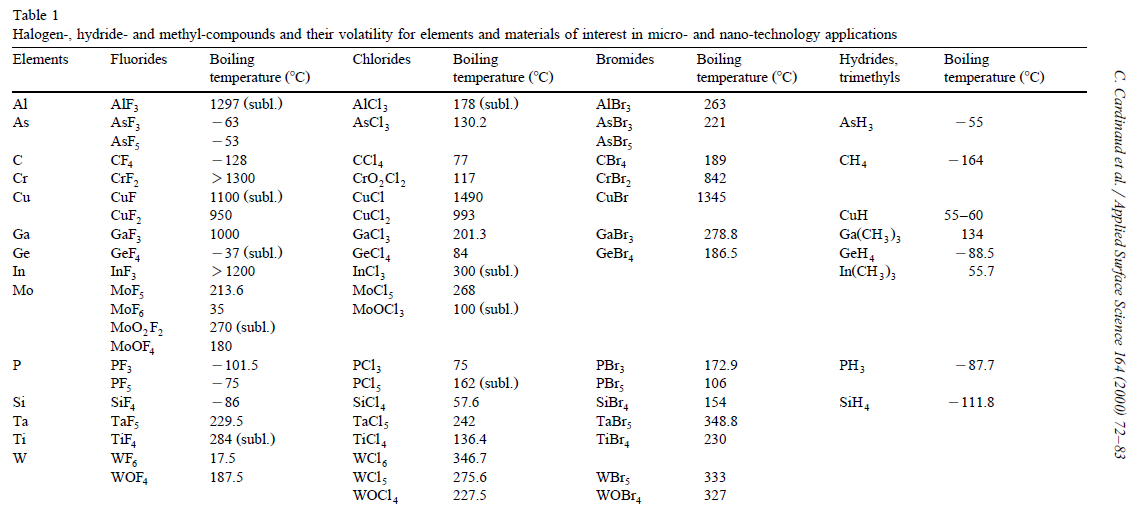
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the "trihalomethane, haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry group, symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20M kg/y are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with Hydrogen_fluoride, HF: :CHCl3 + 3 HF → CHF3 + 3 HCl It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of Mercury(II) fluoride, mercury fluoride and calcium fluoride. The exchange reaction works with iodofo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FE-13
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the "trihalomethane, haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry group, symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20M kg/y are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with Hydrogen_fluoride, HF: :CHCl3 + 3 HF → CHF3 + 3 HCl It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of Mercury(II) fluoride, mercury fluoride and calcium fluoride. The exchange reaction works with iodofo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Ruff
Otto Ruff (12 December 1871 – 17 September 1939) was a German chemist. Life Otto Ruff was born in Schwäbisch Hall, Württemberg. After becoming a pharmacist under the supervision of Carl Magnus von Hell (known from the Hell-Volhard-Zelinsky halogenation) at the University of Stuttgart he joined the group of Hermann Emil Fischer at the University of Berlin. Fischer was noted for his work on carbohydrates (sugars) and so Ruff started his career as an organic chemist. In 1898 he published his work on the transformation of d-Glucose to d-Arabinose, later called the Ruff degradation. Supported by the far-sighted Fischer, who recognized that while organic chemistry was now mature, physical chemistry was growing rapidly, Ruff became head of the new inorganic department in Berlin, working alongside Alfred Stock who was five years his junior. This drastic change in subject benefited Ruff during his work on chlorides sulfur compounds. In 1902 he married Meta Richter, a pharmacist, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in the development of Delaware and first arose as a major supplier of gunpowder. DuPont developed many polymers such as Vespel, neoprene, nylon, Corian, Teflon, Mylar, Kapton, Kevlar, Zemdrain, M5 fiber, Nomex, Tyvek, Sorona, Corfam and Lycra in the 20th century, and its scientists developed many chemicals, most notably Freon (chlorofluorocarbons), for the refrigerant industry. It also developed synthetic pigments and paints including ChromaFlair. In 2015, DuPont and the Dow Chemical Company agreed to a reorganization plan in which the two companies would merge and split into three. As a merged entity, DuPont simultaneously acquired Dow and renamed itself to DowDuPont on August 31, 2017, and after 18 months spin off the merged entity' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoromethane
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, high global warming potential, and long atmospheric lifetime. Preparation It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: CCl4 + 3HF → CClF3 + 3HCl This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4). Production phaseout Per the international Montreal Protocol, CFC-13 began a phase out and replacement with alternative substances starting in the early 1990s that will culminate in a global ban on its production. The atmospheric abundance of CFC-13 rose from 3.0 parts per trillion (ppt) in year 2010 to 3.3 ppt in year 2020 based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change. Refrigerants are used in a Direct Expansion (DX) system to transfer energy from one environment to another, typically from inside a building to outside (or vice versa) commonly known as an "air conditioner" or "heat pump". Refrigerants can carry per kg 10 times more energy than water and 50 times more than air. Refrigerants are controlled substances due to 1) High Pressures (100-145 psi), 2) Extreme temperatures (-50°C to 145°C), 3) Flammability A1 class non-flammable, A2/A2L class flammable & A3 class extremely flammable/explosive and 4) Toxicity B1-low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Nitride
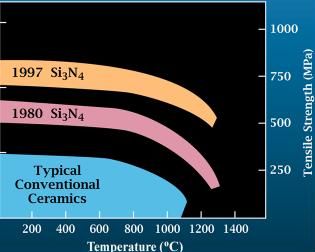
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot . It is very hard (8.5 on the mohs scale). It has a high thermal stability with strong optical nonlinearities for all-optical applications. Production Silicon nitride is prepared by heating powdered silicon between 1300 °C and 1400 °C in a nitrogen atmosphere: :3 Si + 2 → The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected. In addition to , several other silicon nitride phases (with chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Oxide
Silicon oxide may refer to either of the following: *Silicon dioxide or quartz, SiO2, very well characterized *Silicon monoxide Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common o ..., SiO, not very well characterized {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Etching
Plasma etching is a form of plasma processing used to fabricate integrated circuits. It involves a high-speed stream of glow discharge (plasma) of an appropriate gas mixture being shot (in pulses) at a sample. The plasma source, known as etch species, can be either charged (ions) or neutral (atoms and radicals). During the process, the plasma generates volatile etch products at room temperature from the chemical reactions between the elements of the material etched and the reactive species generated by the plasma. Eventually the atoms of the shot element embed themselves at or just below the surface of the target, thus modifying the physical properties of the target. Mechanisms Plasma generation A plasma is a high energetic condition in which a lot of processes can occur. These processes happen because of electrons and atoms. To form the plasma electrons have to be accelerated to gain energy. Highly energetic electrons transfer the energy to atoms by collisions. Three different pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industry (economics)
In macroeconomics, an industry is a branch of an economy that Production (economics) , produces a closely-related set of raw materials, Good (economics) , goods, or Service (economics) , services. For example, one might refer to the wood industry or to the insurance industry. When evaluating a single group or company, its dominant source of revenue is typically used by industry classifications to classify it within a specific industry. For example the International Standard Industrial Classification (ISIC) – used directly or through derived classifications for the official statistics of most countries worldwide – classifies "statistical units" by the "economic activity in which they mainly engage". Industry is then defined as "set of statistical units that are classified into the same ISIC category". However, a single business need not belong just to one industry, such as when a large business (often referred to as a conglomerate (company), conglomerate) Diversification (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glass. Its electrical resistivity and conductivity, resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping (semiconductor), doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Trifluoride
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry. Preparation and structure In solid SbF3, the Sb centres have octahedral molecular geometry and are linked by bridging fluoride ligands. Three Sb–F bonds are short (192 pm) and three are long (261 pm). Because it is a polymer, SbF3 is far less volatile than related compounds AsF3 and SbCl3. SbF3 is prepared by treating antimony trioxide with hydrogen fluoride: :Sb2O3 + 6 HF → 2 SbF3 + 3 H2O The compound is a mild Lewis acid, hydrolyzing slowly in water. With fluorine, it is oxidized to give antimony pentafluoride. :SbF3 + F2 → SbF5 Applications It is used as a fluorination reagent in organic chemistry. This application was reported by the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromide. Currently its main use is as a laboratory reagent. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Uses Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent. Bromoform also has medical uses; injections of bromoform are sometimes used instead of epinephrine to treat severe asthma cases. Bromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |