|
Flory–Schulz Distribution
The Flory–Schulz distribution is a discrete probability distribution named after Paul Flory and Günter Victor Schulz that describes the relative ratios of polymers of different length that occur in an ideal step-growth polymerization process. The probability mass function (pmf) for the mass fraction of chains of length k is: w_a(k) = a^2 k (1-a)^\text In this equation, ''k'' is the number of monomers in the chain, and ''0 |
Real Number
In mathematics, a real number is a number that can be used to measure a continuous one- dimensional quantity such as a duration or temperature. Here, ''continuous'' means that pairs of values can have arbitrarily small differences. Every real number can be almost uniquely represented by an infinite decimal expansion. The real numbers are fundamental in calculus (and in many other branches of mathematics), in particular by their role in the classical definitions of limits, continuity and derivatives. The set of real numbers, sometimes called "the reals", is traditionally denoted by a bold , often using blackboard bold, . The adjective ''real'', used in the 17th century by René Descartes, distinguishes real numbers from imaginary numbers such as the square roots of . The real numbers include the rational numbers, such as the integer and the fraction . The rest of the real numbers are called irrational numbers. Some irrational numbers (as well as all the rationals) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
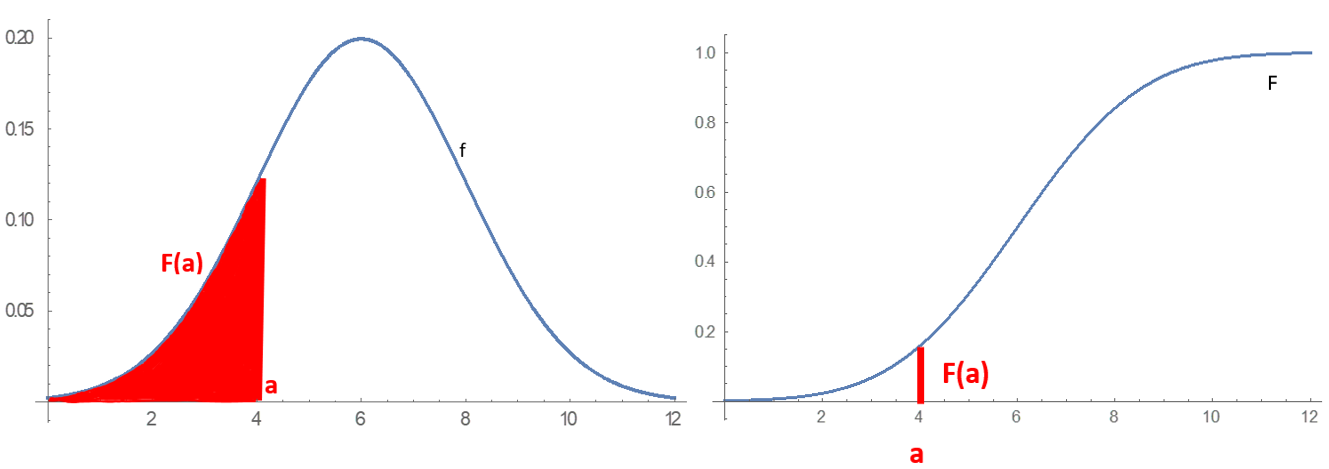
Probability Distribution
In probability theory and statistics, a probability distribution is a Function (mathematics), function that gives the probabilities of occurrence of possible events for an Experiment (probability theory), experiment. It is a mathematical description of a Randomness, random phenomenon in terms of its sample space and the Probability, probabilities of Event (probability theory), events (subsets of the sample space). For instance, if is used to denote the outcome of a coin toss ("the experiment"), then the probability distribution of would take the value 0.5 (1 in 2 or 1/2) for , and 0.5 for (assuming that fair coin, the coin is fair). More commonly, probability distributions are used to compare the relative occurrence of many different random values. Probability distributions can be defined in different ways and for discrete or for continuous variables. Distributions with special properties or for especially important applications are given specific names. Introduction A prob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Flory
Paul John Flory (June 19, 1910 – September 9, 1985) was an American chemist and Nobel laureate who was known for his work in the field of polymers, or macromolecules. He was a pioneer in understanding the behavior of polymers in solution, and won the Nobel Prize in Chemistry in 1974 "for his fundamental achievements, both theoretical and experimental, in the physical chemistry of macromolecules". Biography Personal life Flory was born in Sterling, Illinois, on June 19, 1910 to Ezra Flory and Martha Brumbaugh. His father worked as a clergyman-educator, and his mother was a school teacher. His ancestors were German Huguenots, who traced their roots back to Alsace. He first gained an interest in science from Carl W Holl, who was a chemistry professor at Manchester College. In 1936, he married Emily Catherine Tabor. They had three children together: Susan Springer, Melinda Groom and Paul John Flory, Jr. His first position was at DuPont with Wallace Carothers. He was posthumou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Günter Victor Schulz
Günter Victor Schulz (born October 4, 1905, in Łódź; died February 25, 1999, in Mainz) was a German chemist. He made seminal contributions to macromolecular chemistry. His name lives on in the Flory-Schulz distribution and the Schulz-Zimm distribution. Literature * August Ludwig Degener, Walter Habel: '' Wer ist wer? Das deutsche Who's Who, Band 16.'', Arani, Berlin, 1970 ISBN 3-7605-2007-3, S. 1202. * Werner Schuder (Hrsg.): ''Kürschners Deutscher Gelehrten-Kalender ''Kürschners Deutscher Gelehrten-Kalender'' (English: "Kürschner's Encyclopedia of German Scholars"), formerly subtitled ''Lexikon der lebenden deutschsprachigen Wissenschaftler'' ("Encyclopedia of Living German-Speaking Scholars"), is a German ....'' Band 3. 13. Ausgabe. De Gruyter, Berlin/New York 1980, ISBN 3-110-07434-6. S. 3580. References {{DEFAULTSORT:Schulz, Gunter 1905 births 1999 deaths German chemists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PVC), which are produced in high tonnages each year due to their usefulnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Probability Mass Function
In probability and statistics, a probability mass function (sometimes called ''probability function'' or ''frequency function'') is a function that gives the probability that a discrete random variable is exactly equal to some value. Sometimes it is also known as the discrete probability density function. The probability mass function is often the primary means of defining a discrete probability distribution, and such functions exist for either scalar or multivariate random variables whose domain is discrete. A probability mass function differs from a continuous probability density function (PDF) in that the latter is associated with continuous rather than discrete random variables. A continuous PDF must be integrated over an interval to yield a probability. The value of the random variable having the largest probability mass is called the mode. Formal definition Probability mass function is the probability distribution of a discrete random variable, and provides the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt.%'' or ''% w/w''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Distribution
In probability theory and statistics, the geometric distribution is either one of two discrete probability distributions: * The probability distribution of the number X of Bernoulli trials needed to get one success, supported on \mathbb = \; * The probability distribution of the number Y=X-1 of failures before the first success, supported on \mathbb_0 = \ . These two different geometric distributions should not be confused with each other. Often, the name ''shifted'' geometric distribution is adopted for the former one (distribution of X); however, to avoid ambiguity, it is considered wise to indicate which is intended, by mentioning the support explicitly. The geometric distribution gives the probability that the first occurrence of success requires k independent trials, each with success probability p. If the probability of success on each trial is p, then the probability that the k-th trial is the first success is :\Pr(X = k) = (1-p)^p for k=1,2,3,4,\dots The above form of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer–Tropsch Process
The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons. In the usual implementation, carbon monoxide and hydrogen, the feedstocks for FT, are produced from coal, natural gas, or biomass in a process known as gasification. The process then converts these gases into synthetic lubrication oil and synthetic fuel. This process has received intermittent attention as a source of low-sulfur diesel fuel and to address the supply or cost of petroleum-derived hydrocarbons. Fischer–Tropsch process is discussed as a step of producing carbon-neutral liquid hydrocarbon fuels from CO2 and hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Fuel
Liquid fuels are combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container. It is the fumes of liquid fuels that are flammable instead of the fluid. Most liquid fuels in widespread use are derived from fossil fuels; however, there are several types, such as hydrogen fuel (for automotive uses), ethanol, and biodiesel, which are also categorized as a liquid fuel. Many liquid fuels play a primary role in transportation and the economy. Liquid fuels are contrasted with solid fuels and gaseous fuels. General properties Some common properties of liquid fuels are that they are easy to transport, and can be handled with relative ease. Physical properties of liquid fuels vary by temperature, though not as greatly as for gaseous fuels. Some of these properties are: flash point, the lowest temperature at which a flammable concentration of vapor is produced; fire point, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |