|
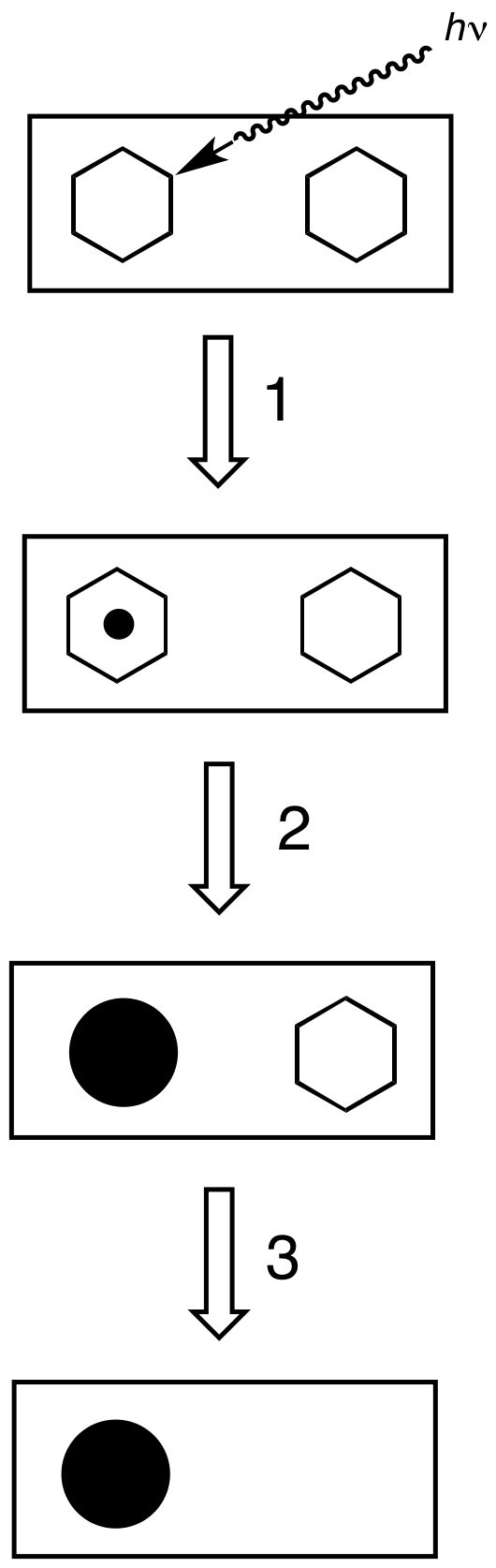
Film Developing
Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. All processes based upon the gelatin silver process are similar, regardless of the film or paper's manufacturer. Exceptional variations include instant films such as those made by Polaroid and thermally developed films. Kodachrome required Kodak's proprietary K-14 process. Kodachrome film production ceased in 2009, and K-14 processing is no longer available as of December 30, 2010. Ilfochrome materials use the dye destruction process. Deliberately using the wrong process for a film is known as cross processing. Common processes All photograp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine the sensitivity, contrast, and resolution of the film. The emulsion will gradually darken if left exposed to light, but the process is too slow and incomplete to be of any practical use. Instead, a very short exposure to the image formed by a camera lens is used to produce only a very slight chemical change, proportional to the amount of light absorbed by each crystal. This creates an invisible latent image in the emulsion, which can be chemically developed into a visible photograph. In addition to visible light, all films are sensitive to ultraviolet light, X-rays, gamma rays, and high-energy particles. Unmodified silver halide crystals are sensitive only to the blue part of the visible spectrum, producing unnatural-looking rendit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Processing
Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. All processes based upon the gelatin silver process are similar, regardless of the film or paper's manufacturer. Exceptional variations include instant films such as those made by Polaroid and thermally developed films. Kodachrome required Kodak's proprietary K-14 process. Kodachrome film production ceased in 2009, and K-14 processing is no longer available as of December 30, 2010. Ilfochrome materials use the dye destruction process. Deliberately using the wrong process for a film is known as cross processing. Common processes All p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wetting Agent
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-ionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypo Clear
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2 SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air. Preparation Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a white solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling. :SO2 + 2 NaOH -> Na2SO3 + H2O Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate. The overall reaction is: :SO2 + Na2CO3 -> Na2SO3 + CO2 Applications Sodium sulfite is primarily used in the pulp and paper industry. It has been also applied in the thermomechanical conversion of wood to fibres (''defibration'') for producing medium density fibreboards (M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Thiosulfate
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound with the formula . It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether. Production It is produced by treating ammonium sulfite with sulfur at temperatures between 85 and 110 °C: : Applications Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide): : : Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation. The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes derived from sodium thiosulfate. Other Ammonium thiosulfate can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Halide
A silver halide (or silver salt) is one of the chemical compounds that can form between the Chemical element, element silver (Ag) and one of the halogens. In particular, bromine (Br), chlorine (Cl), iodine (I) and fluorine (F) may each combine with silver to produce silver bromide (AgBr), silver chloride (AgCl), silver iodide (AgI), and three forms of silver fluoride, respectively. As a group, they are often referred to as the silver halides, and are often given the pseudo-chemical notation AgX. Although most silver halides involve silver atoms with oxidation states of +1 (Ag+), silver halides in which the silver atoms have oxidation states of +2 (Ag2+) are known, of which silver(II) fluoride is the only known stable one. Silver halides are light-sensitive chemicals, and are commonly used in photographic film and paper. Applications Light sensitivity Silver halides are used in photographic film and photographic paper, including graphic art film and paper, where silver halide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Fixer
Photographic fixer is a mix of chemicals used in the final step in the photographic processing of film or paper. The fixer stabilises the image, removing the unexposed silver halide remaining on the photographic film or photographic paper, leaving behind the reduced metallic silver that forms the image. By fixation, the film or paper is insensitive to further action by light. Without fixing, the remaining silver halide would darken and cause fogging of the image. Fixation is commonly achieved by treating the film or paper with a solution of thiosulfate salt. Popular salts are sodium thiosulfate—commonly called hypo—and ammonium thiosulfate—commonly used in modern rapid fixer formulae. Fixation involves these chemical reactions (X = halide, typically Br−):Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. :AgX + 2 S2O32− → g(S2O3)2sup>3− + X− :AgX + 3 S2O32− → g(S2O3)3sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global demand for aceti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stop Bath
Stop bath is a acidic solution used for processing black-and-white photographic films, plates, and paper. It is used to neutralize the alkaline developer, thus halting development. Stop bath is commonly a 2% dilution of acetic acid in water, though a 2.5% solution of potassium or sodium metabisulfite works just as well. Because organic developers only work in alkaline solutions, stop bath halts the development process almost immediately and provides precise control of development time. Neutralizing the alkalinity of basic developers also helps to preserve the strength of the fixer, making it last longer. Stop bath accounts for the vinegar-like odor of the darkroom. In its concentrated form it can cause chemical burns, but is harmless when diluted to a working solution. Stop bath becomes exhausted when carried over developer causes the solution to become alkaline. For indicator stop baths, which changes color to indicate when the bath is exhausted and no longer effective, a pH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |