|
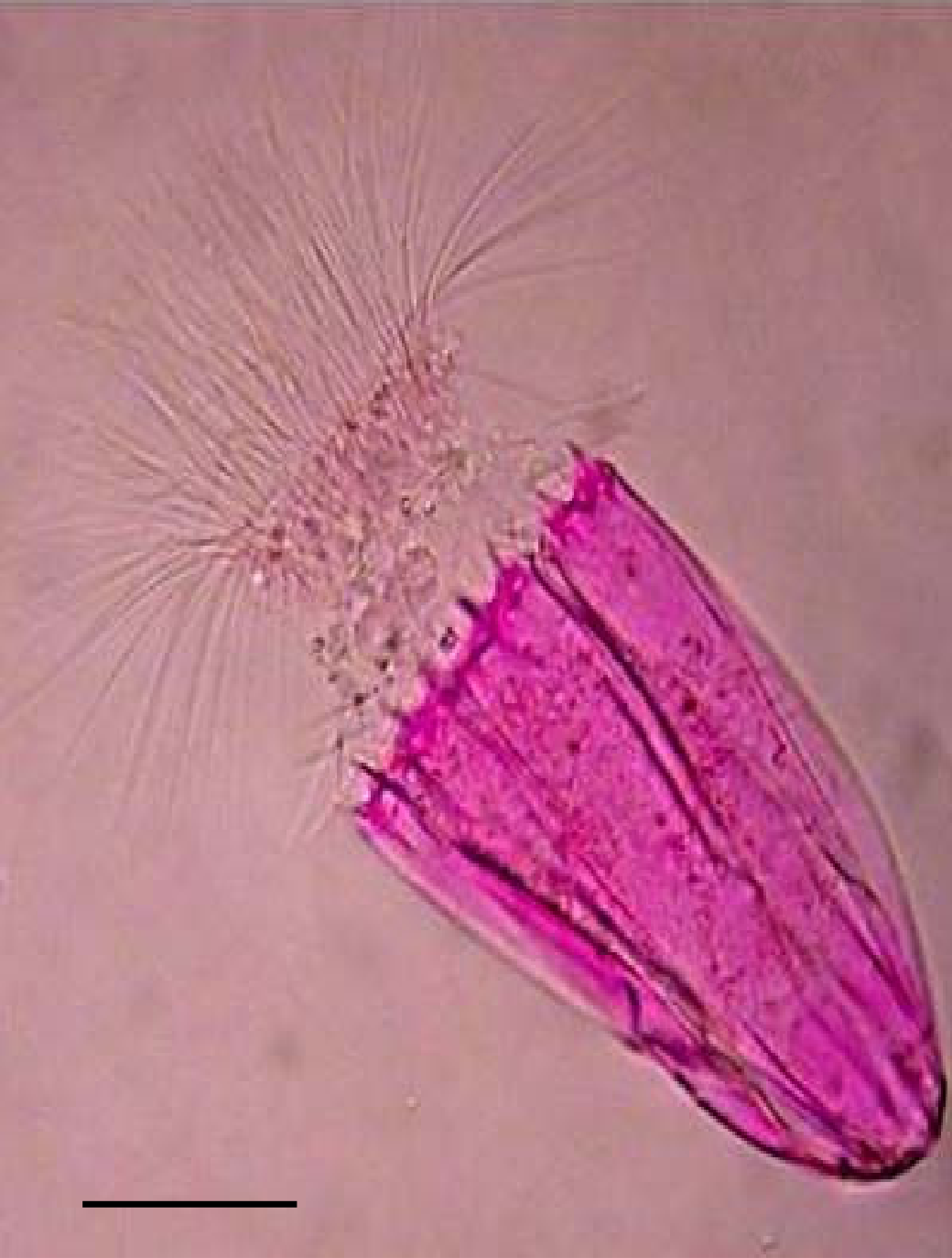
Ferroglobus Placidus
''Ferroglobus'' is a genus of the Archaeoglobaceae.See the NCBIbr>webpage on Ferroglobus Data extracted from the ''Ferroglobus'' is a hyperthermophilic genus phylogenetically located within the Euryarchaeota. It consists of one species, ''F. placidus'', isolated from hydrothermal vent sediment off the coast of Italy. ''F. placidus'' grows best at 85 °C and a neutral pH. It cannot grow at temperatures below 65 °C or above 95 °C. Cells possess an S-layer cell wall and archaella. Metabolically, ''Ferroglobus'' is quite unique compared to its relative ''Archaeoglobus''. ''F. placidus'' was the first hyperthermophile discovered to grow anaerobically by oxidizing aromatic compounds such as benzoate coupled to the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+). Hydrogen gas (H2) and sulfide (H2S) can also be used as energy sources. Due to its anaerobic lifestyle, nitrate (NO3−) is used as a terminal electron acceptor whereby it is converted to nitri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebacteria kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from the other two domains, Bacteria and Eukaryota. Archaea are further divided into multiple recognized phyla. Classification is difficult because most have not been isolated in a laboratory and have been detected only by their gene sequences in environmental samples. Archaea and bacteria are generally similar in size and shape, although a few archaea have very different shapes, such as the flat, square cells of ''Haloquadratum walsbyi''. Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably for the enzymes involved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaerobe
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular (e.g. protozoans, bacteria) or multicellular. Most fungi are obligate aerobes, requiring oxygen to survive. However, some species, such as the Chytridiomycota that reside in the rumen of cattle, are obligate anaerobes; for these species, anaerobic respiration is used because oxygen will disrupt their metabolism or kill them. Deep waters of the ocean are a common anoxic environment. First observation In his letter of 14 June 1680 to The Royal Society, Antonie van Leeuwenhoek described an experiment he carried out by filling two identical glass tubes about halfway with crushed pepper powder, to which some clean rain water was added. Van Leeuwenhoek sealed one of the glass tubes using a flame and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Springer Verlag
Springer Science+Business Media, commonly known as Springer, is a German multinational publishing company of books, e-books and peer-reviewed journals in science, humanities, technical and medical (STM) publishing. Originally founded in 1842 in Berlin, it expanded internationally in the 1960s, and through mergers in the 1990s and a sale to venture capitalists it fused with Wolters Kluwer and eventually became part of Springer Nature in 2015. Springer has major offices in Berlin, Heidelberg, Dordrecht, and New York City. History Julius Springer founded Springer-Verlag in Berlin in 1842 and his son Ferdinand Springer grew it from a small firm of 4 employees into Germany's then second largest academic publisher with 65 staff in 1872.Chronology ". Springer Science+Business Media. In 1964, Springer expanded its business internationally, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Prentice Hall
Prentice Hall was an American major educational publisher owned by Savvas Learning Company. Prentice Hall publishes print and digital content for the 6–12 and higher-education market, and distributes its technical titles through the Safari Books Online e-reference service. History On October 13, 1913, law professor Charles Gerstenberg and his student Richard Ettinger founded Prentice Hall. Gerstenberg and Ettinger took their mothers' maiden names, Prentice and Hall, to name their new company. Prentice Hall became known as a publisher of trade books by authors such as Norman Vincent Peale; elementary, secondary, and college textbooks; loose-leaf information services; and professional books. Prentice Hall acquired the training provider Deltak in 1979. Prentice Hall was acquired by Gulf+Western in 1984, and became part of that company's publishing division Simon & Schuster. S&S sold several Prentice Hall subsidiaries: Deltak and Resource Systems were sold to National Education ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Archaea Genera
This article lists the genera of the Archaea. The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN) and National Center for Biotechnology Information (NCBI). Phylogeny National Center for Biotechnology Information (NCBI) taxonomy was initially used to decorate the genome tree via tax2tree. The 16S rRNA-based Greengenes taxonomy is used to supplement the taxonomy particularly in regions of the tree with no cultured representatives. List of Prokaryotic names with Standing in Nomenclature (LPSN) is used as the primary taxonomic authority for establishing naming priorities. Taxonomic ranks are normalised using phylorank and the taxonomy manually curated to remove polyphyletic groups. Cladogram was taken from the GTDB release 07-RS207 (8th April 2022). The position of clades with a "question mark" are based on the additional phylogeny of the 16S rRNA-based LTP_12_2021 by The All-Species Living Tree Project. Phylum " Altarcha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Banded Iron Formation
Banded iron formations (also known as banded ironstone formations or BIFs) are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness and extend laterally for several hundred kilometers. Almost all of these formations are of Precambrian age and are thought to record the Great Oxygenation Event, oxygenation of the Earth's oceans. Some of the Earth's oldest rock formations, which formed about (Year#SI prefix multipliers, Ma), are associated with banded iron formations. Banded iron formations are thought to have formed in sea water as the result of oxygen production by photosynthesis, photosynthetic cyanobacteria. The oxygen combined with dissolved iron in Earth's oceans to form insoluble iron oxides, which precipitated out, forming a thin layer on the ocean floor. Each band is similar to a varve, resulting from cyclic variations in oxygen production. Banded iron formation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e.g. sodium thiosulfate . Thiosulfate also refers to the esters of thiosulfuric acid, e.g. ''O'',''S''-dimethyl thiosulfate . The prefix thio- indicates that the thiosulfate is a sulfate with one oxygen replaced by sulfur. Thiosulfate is tetrahedral at the central S atom. Thiosulfate salts occur naturally. Thiosulfate ion has C3v symmetry, and is produced by certain biochemical processes. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances. Sodium thiosulfate, commonly called ''hypo'' (from "hyposulfite"), was widely used in photography to fix black and white negatives and prints after the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terminal Electron Acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mistakenly called electron receptors. Typical oxidizing agents undergo permanent chemical alteration through covalent or ionic reaction chemistry, resulting in the complete and irreversible transfer of one or more electrons. In many chemical circumstances, however, the transfer of electronic charge from an electron donor may be only fractional, meaning an electron is not completely transferred, but results in an electron resonance between the donor and acceptor. This leads to the formation of charge transfer complexes in which the components largely retain their chemical identities. The electron accepting power of an acceptor molecule is measured by its electron affinity which is the energy released when filling the lowest unoccupied molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ... with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2−, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + 8 M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)