|
Evapoporometry
Evapoporometry is a method used to determine pore-size in synthetic membranes. Based on the Kelvin equation, this technique is most accurate for detection of pore diameters between 4 nm to 150 nm. Theory Evapoporometry uses modified forms of the Kelvin equation to relate the evaporation of a wetting liquid (usually 2-propanol) from a membrane to the average diameter of the pores in that membrane. The primary equation used in this technique is: d = - Where d is the pore diameter, y is the surface tension, V_\text is the vapor molar volume, R is the gas constant, T is the absolute temperature, W_A is the instantaneous evaporation rate in mol/s, and W^\circ_A is the average evaporation rate of the free-standing liquid layer in mol/s. Method Evapoporometry has the significant advantage of requiring only a lab scale, 2-propanol (or another wetting fluid), and a cell in which to contain the sample and 2-propanol. The sample is immersed for some time in 2-propanol prior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porosimetry
Porosimetry is an analytical technique used to determine various quantifiable aspects of a material's porous structure, such as pore diameter, total pore volume, surface area, and bulk and absolute densities. The technique involves the intrusion of a non-wetting liquid (often mercury) at high pressure into a material through the use of a porosimeter. The pore size can be determined based on the external pressure needed to force the liquid into a pore against the opposing force of the liquid's surface tension. A force balance equation known as Washburn's equation for the above material having cylindrical pores is given as: :P_L - P_G = -\frac :P_ = pressure of liquid :P_ = pressure of gas :\sigma = surface tension of liquid :\theta = contact angle of intrusion liquid :D_ = pore diameter Since the technique is usually performed within a vacuum, the initial gas pressure is zero. The contact angle of mercury with most solids is between 135° and 142°, so an average of 140 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hollow Fiber Membrane
Hollow fiber membranes (HFMs) are a class of artificial membranes containing a semi-permeable barrier in the form of a hollow fiber. Originally developed in the 1960s for reverse osmosis applications, hollow fiber membranes have since become prevalent in water treatment, desalination, cell culture, medicine, and tissue engineering. Most commercial hollow fiber membranes are packed into cartridges which can be used for a variety of liquid and gaseous separations. Manufacturing HFMs are commonly produced using artificial polymers. The specific production methods involved are heavily dependent on the type of polymer used as well as its molecular weight. HFM production, commonly referred to as "spinning," can be divided into four general types: * Melt Spinning, in which a thermoplastic polymer is melted and extruded through a spinneret into air and subsequently cooled. * Dry Spinning, in which a polymer is dissolved in an appropriate solvent and extruded through a spinneret i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Membranes
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century.Pinnau, I., Freeman, B.D., ''Membrane Formation and Modification'', ACS, 1999. A wide variety of synthetic membranes is known.Osada, Y., Nakagawa, T., ''Membrane Science and Technology'', New York: Marcel Dekker, Inc,1992. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin Equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin. Formulation The original form of the Kelvin equation, published in 1871, is: p(r_1 , r_2) = P - \frac \left ( \frac + \frac \right ), where: * p(r) = vapor pressure at a curved interface of radius r * P = vapor pressure at flat interface ( r = \infty ) = p_ * \gamma = surface tension * \rho _ = density of vapor * \rho _ = density of liquid * r_1 , r_2 = radii of curvature along the principal sections of the curved interface. This may be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-propanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume occupied by a substance to the amount of substance, usually given at a given temperature and pressure. It is equal to the molar mass (''M'') divided by the mass density (''ρ''): V_ = \frac . The molar volume has the SI unit of cubic metres per mole (m3/mol), although it is more typical to use the units cubic decimetres per mole (dm3/mol) for gases, and cubic centimetres per mole (cm3/mol) for liquids and solids. Definition The molar volume of a substance ''i'' is defined as its molar mass divided by its density ''ρ''''i''0: V_ = . For an ideal mixture containing ''N'' components, the molar volume of the mixture is the weighted sum of the molar volumes of its individual components. For a real mixture the molar volume cannot be calculated without knowing the density: V_ = \frac. There are many liquid–liquid mixtures, for instance mixing pure ethanol and pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics. Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic work and heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as manifestations of the kinetic energy of free motion of microscopic particles such as atoms, molecules, and electrons. From the thermodynamic viewpoint, for historical reasons, because of how it is defined and measured, this microscopic kinetic definition is regarded as an "empirical" temperature. It was adopted because in practice it can generally be measured more precisely than can Kelvin's thermodynamic temperature. A thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamics. By convention, it is reported on the '' Kelvin scale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
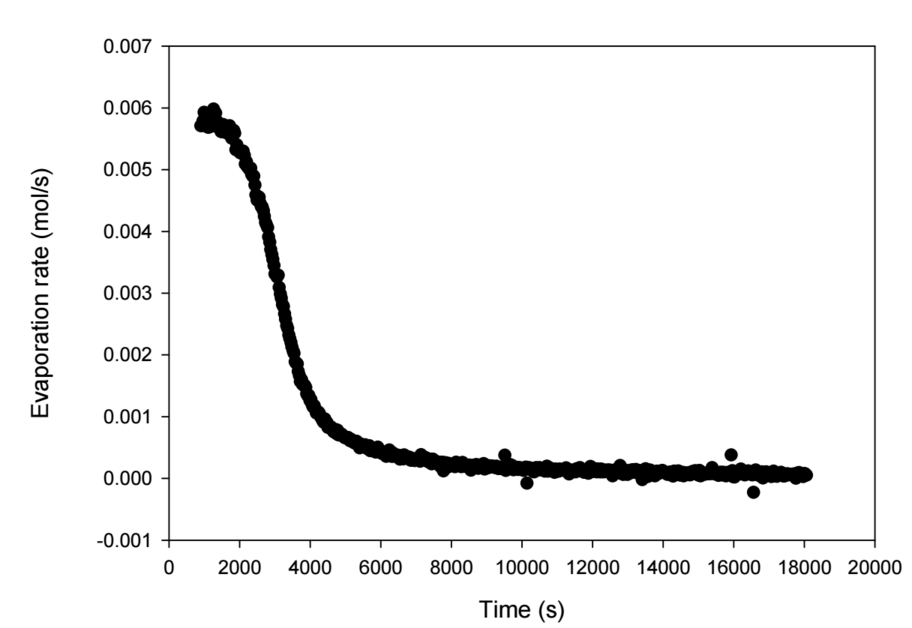
Sample Evapoporometry Curve
Sample or samples may refer to: Base meaning * Sample (statistics), a subset of a population – complete data set * Sample (signal), a digital discrete sample of a continuous analog signal * Sample (material), a specimen or small quantity of something * Sample (graphics), an intersection of a color channel and a pixel * SAMPLE history, a mnemonic acronym for questions medical first responders should ask * Product sample, a sample of a consumer product that is given to the consumer so that he or she may try a product before committing to a purchase * Standard cross-cultural sample, a sample of 186 cultures, used by scholars engaged in cross-cultural studies People *Sample (surname) *Samples (surname) * Junior Samples (1926–1983), American comedian Places * Sample, Kentucky, unincorporated community, United States * Sampleville, Ohio, unincorporated community, United States * Hugh W. and Sarah Sample House, listed on the National Register of Historic Places in Iowa, United ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Balance
An analytical balance (or chemical ''balance'') is a class of balance designed to measure small mass in the sub-milligram range. The measuring pan of an analytical balance (0.1 mg resolution or better) is inside a transparent enclosure with doors so that dust does not collect and so any air currents in the room do not affect the balance's operation. This enclosure is often called a draft shield. The use of a mechanically vented balance safety enclosure, which has uniquely designed acrylic airfoils, allows a smooth turbulence-free airflow that prevents balance fluctuation and the measure of mass down to 1 μg without fluctuations or loss of product. Also, the sample must be at room temperature to prevent natural convection from forming air currents inside the enclosure from causing an error in reading. Single pan mechanical substitution balance is a method of maintaining consistent response throughout the useful capacity of the balance. This is achieved by maintaining a constan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Membrane
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century.Pinnau, I., Freeman, B.D., ''Membrane Formation and Modification'', ACS, 1999. A wide variety of synthetic membranes is known.Osada, Y., Nakagawa, T., ''Membrane Science and Technology'', New York: Marcel Dekker, Inc,1992. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)


