|
Etelcalcetide
Etelcalcetide (formerly velcalcetide, trade name Parsabiv) is a calcimimetic drug for the treatment of secondary hyperparathyroidism in patients undergoing hemodialysis. It is administered intravenously at the end of each dialysis session. Etelcalcetide functions by binding to and activating the calcium-sensing receptor in the parathyroid gland. Parsabiv is currently owned by Amgen and Ono Pharmaceuticals in Japan. Medical uses Etelcalcetide is used for the treatment of secondary hyperparathyroidism in people with chronic kidney disease (CKD) on hemodialysis. Hyperparathyroidism is the condition of elevated parathyroid hormone (PTH) levels and is often observed in people with CKD. Pharmacodynamics Mechanism of action Etelcalcetide functions by binding to and activating the calcium-sensing receptor (CaSR) in the parathyroid gland as an allosteric activator, resulting in PTH reduction and suppression. Pharmacokinetics Etelcalcetide functions in a first order elimination, with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcimimetic
A calcimimetic is a pharmaceutical drug that mimics the action of calcium on tissues, by allosteric activation of the calcium-sensing receptor that is expressed in various human organ tissues. Calcimimetics are used to treat secondary hyperparathyroidism (SHPT). In the treatment of SHPT patients on dialysis calcimimetics does not appear to affect the risk of early death. It does decrease the need for a parathyroidectomy but caused more issues with low blood calcium levels and vomiting. Cinacalcet was the first calcimimetic to be approved. Cinacalcet mimics calcium at the parathyroid hormone receptor. This binding will increase the sensitivity of calcium-sensing receptors (CaSR) on the parathyroid gland. As a result of the receptor "thinking" there is sufficient calcium, parathyroid hormone (PTH) secretion will be reduced. Lower calcium levels will be seen as well. On August 25, 2015 Amgen Inc. announced that it had submitted a new drug application with the United States Food and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous Injection
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consump ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat. Over 30 definitions of nausea were proposed in a 2011 book on the topic. Nausea is a non-specific symptom, which means that it has many possible causes. Some common causes of nausea are gastroenteritis and other gastrointestinal disorders, food poisoning, motion sickness, dizziness, migraine, fainting, low blood sugar, anxiety, and lack of sleep. Nausea is a side effect of many medications including chemotherapy, or morning sickness in early pregnancy. Nausea may also be caused by disgust and depression. Medications taken to prevent and treat nausea and vomiting are called antiemetics. The most commonly prescribed antiemetics in the US are promethazine, metoclopramide, and the newer ondansetron. The word nausea is from Lat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amgen Inc
Amgen Inc. (formerly Applied Molecular Genetics Inc.) is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, Amgen was established in Thousand Oaks, California, in 1980.Baker, Pam (2002). ''Thousand Oaks Westlake Village: A Contemporary Portrait''. Community Communications, Inc. Page 37. . Amgen's Thousand Oaks staff in 2017 numbered 5,125 (7.5% of total city employment) and included hundreds of scientists, making Amgen the largest employer in Ventura County. Focused on molecular biology and biochemistry, its goal is to provide a healthcare business based on recombinant DNA technology. In 2018, the company's largest selling product lines were Neulasta, an immunostimulator used to prevent infections in patients undergoing cancer chemotherapy and Enbrel, a tumor necrosis factor blocker used in the treatment of rheumatoid arthritis and other autoimmune diseases. Other product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ChemSpider
ChemSpider is a database of chemicals. ChemSpider is owned by the Royal Society of Chemistry. Database The database contains information on more than 100 million molecules from over 270 data sources including: * EPA DSSTox * U.S. Food and Drug Administration (FDA) * Human Metabolome Database * Journal of Heterocyclic Chemistry * KEGG * KUMGM * LeadScope * LipidMAPS * Marinlit * MDPI * MICAD * MLSMR * MMDB * MOLI * MTDP * Nanogen * Nature Chemical Biology * NCGC * NIAID * National Institutes of Health (NIH) * NINDS Approved Drug Screening Program * NIST * NIST Chemistry WebBook * NMMLSC * NMRShiftDB * PANACHE * PCMD * PDSP * Peptides * Prous Science Drugs of the Future * QSAR * R&D Chemicals * San Diego Center for Chemical Genomics * SGCOxCompounds, SGCStoCompounds * SMID * Specs * Structural Genomics Consortium * SureChem * Synthon-Lab * Thomson Pharma * Total TOSLab Building-Blocks * UM-BBD * UPCMLD * UsefulChem * Web of Science * xPharm Each chemical is given a unique i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
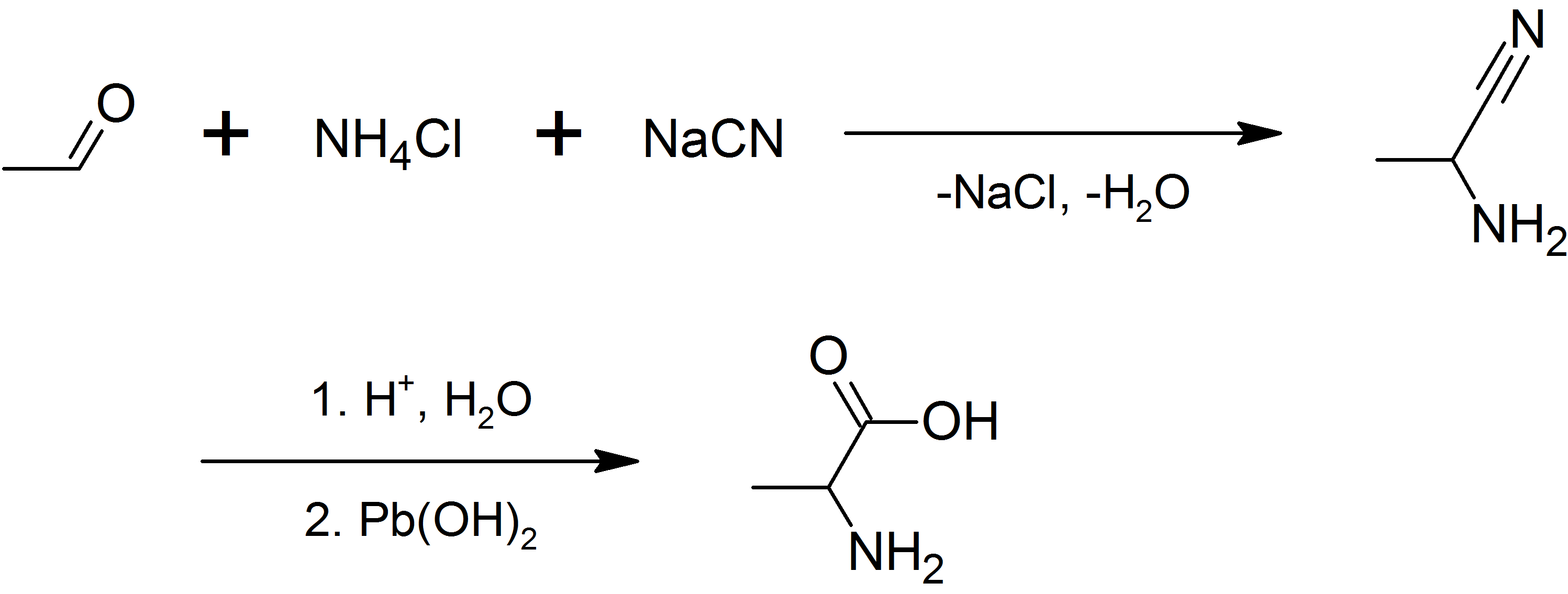
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystein
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |