|
Eluotropic Series
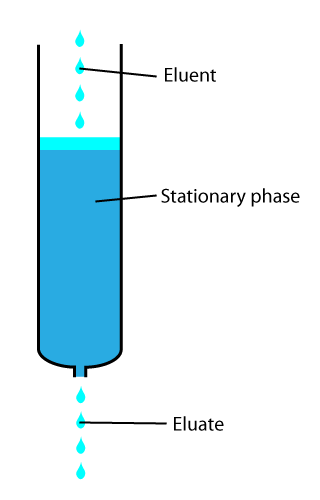
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions. In a liquid chromatography experiment, for example, an analyte is generally adsorbed, or "bound to", an adsorbent in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemists. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handbook'' as an incentive for any purchase of a dozen aprons. Since then the ''Rubber Handbook'' has evolved into the CRC's flagship book, the ''CRC Handbook of Chemistry and Physics''. In 1964, Chemical Rubber decided to focus on its publishing ventures, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaching (chemistry)
Leaching is the process of a solute becoming detached or extracted from its carrier substance by way of a solvent. Leaching is a naturally occurring process which scientists have adapted for a variety of applications with a variety of methods. Specific extraction methods depend on the soluble characteristics relative to the sorbent material such as concentration, distribution, nature, and size. Leaching can occur naturally seen from plant substances (inorganic and organic), solute leaching in soil, and in the decomposition of organic materials. Leaching can also be applied affectedly to enhance water quality and contaminant removal, as well as for disposal of hazardous waste products such as fly ash, or rare earth elements (REEs). Understanding leaching characteristics is important in preventing or encouraging the leaching process and preparing for it in the case where it is inevitable. In an ideal leaching equilibrium stage, all the solute is dissolved by the solvent, leaving th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gradient Elution
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column. HPLC has been used for manufacturing (''e.g.'', during the production process of pharmaceutical and biological products), legal (''e.g.'', detecting performance enhancement drugs in urine), research (''e.g.'', separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (''e.g.'', detecting vitamin D levels in blood serum) purposes. Chrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desorption
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface. There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there is no one equation that describes the process. Note that desorption is the opposite of adsorption, which differs from absorption because it refers to substances being stuck to the surface, as opposed to being absorbed into the bulk. Desorption can occur after a reaction between a catalyst and an adsorbed compound; or during stripping or chromatography which are types of separation processes. Desorption mechanisms Depending on the nature of the adsorbent-to-surface bond, there are a multitude of mechanisms for desorption. The surface bond of a sorbant can be cleaved thermally, through chemical react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Blood Cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "hollow vessel", with ''-cyte'' translated as "cell" in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries. The cytoplasm of a red blood cell is rich in hemoglobin, an iron-containing biomolecule that can bind oxygen and is responsible for the red color of the cells and the blood. Each human red blood cell contains approximately 270 million hemoglobin molecules. The cell membrane is composed of proteins and lipids, and this structure provides properties essential for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion-exchange Chromatography
Ion chromatography (or ion-exchange chromatography) separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein. The two types of ion chromatography are anion-exchange and cation-exchange. Cation-exchange chromatography is used when the molecule of interest is positively charged. The molecule is positively charged because the pH for chromatography is less than the pI (a/k/a pH(I)). In this type of chromatography, the stationary phase is negatively charged and positively charged molecules are loaded to be attracted to it. Anion-exchange chromatography is when the stationary phase is positively charged and negatively charged molecules (meaning that pH for chromatography is greater than the pI) are loaded to be attracted to it. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stationary Phase (chemistry)
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The purpose of preparati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |