|
Ellestadite
Fluorellestadite is a rare nesosilicate of calcium, with sulfate and fluorine, with the chemical formula Ca10(SiO4)3(SO4)3F2. It is a member of the apatite group, and forms a series with hydroxylellestadite. Etymology The mineral was originally named wilkeite by Eakle and Rogers in 1914, in honor of R. M. Wilke, a mineral collector and dealer. In 1922, a sample of “wilkeite” was analysed and found to be sufficiently different from the material reported by Eakle and Rogers to consider it a new species.Duncan McConnell (1937The substitution of SiO4 – and SO4 – groups for PO4 -groups in the apatite structure; ellestadite, the end memberAmerican Mineralogist 22: 977–986 The name “ellestadite” was proposed, in honor of Reuben B Ellestad (1900–1993), an American analytic chemist from the Laboratory for Rock Analysis, University of Minnesota, US. In 1982 Rouse and Dunn showed that the Si:S ratio was close to 1:1, giving the formula Ca10(SiO4)3(SO4)3X2, where X repr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nesosilicates
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is generally an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage."Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica." Diamond and graphite provide examples of cleavage. Both are composed solely of a single element, carbon. But in diamond, each carbon atom is bonded to four others in a tetrahedral pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lustre (mineralogy)
Lustre (British English) or luster (American English; see spelling differences) is the way light interacts with the surface of a crystal, rock, or mineral. The word traces its origins back to the Latin ''lux'', meaning "light", and generally implies radiance, gloss, or brilliance. A range of terms are used to describe lustre, such as ''earthy'', ''metallic'', ''greasy'', and ''silky''. Similarly, the term ''vitreous'' (derived from the Latin for glass, ''vitrum'') refers to a glassy lustre. A list of these terms is given below. Lustre varies over a wide continuum, and so there are no rigid boundaries between the different types of lustre. (For this reason, different sources can often describe the same mineral differently. This ambiguity is further complicated by lustre's ability to vary widely within a particular mineral species). The terms are frequently combined to describe intermediate types of lustre (for example, a "vitreous greasy" lustre). Some minerals exhibit unus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streak (mineralogy)
The streak of a mineral is the color of the powder produced when it is dragged across an un-weathered surface. Unlike the apparent color of a mineral, which for most minerals can vary considerably, the trail of finely ground powder generally has a more consistent characteristic color, and is thus an important diagnostic tool in mineral identification. If no streak seems to be made, the mineral's streak is said to be white or colorless. Streak is particularly important as a diagnostic for opaque and colored materials. It is less useful for silicate minerals, most of which have a white streak or are too hard to powder easily. The apparent color of a mineral can vary widely because of trace impurities or a disturbed macroscopic crystal structure. Small amounts of an impurity that strongly absorbs a particular wavelength can radically change the wavelengths of light that are reflected by the specimen, and thus change the apparent color. However, when the specimen is dragged to produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for Lime (material), lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeology, archaeologists and stone trade pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prism (geology)
In sedimentology, a prism is a long, narrow, wedge-shaped sedimentary body. These types of sediments are typically formed during orogenic deformation; for example, the arkose detrital sedimentary rock found in fault troughs. In mineralogy, prismatic is also type of mineral habit (appearance of a crystal). Prismatic minerals have crystals that show a uniform cross-section. Prismatic crystals typically have 3, 4, 6, 8 or 12 faces which are parallel to a crystallographic axis. Klein, Cornelis and Cornelius S. Hurlbut, Jr., ''Manual of Mineralogy,'' Wiley 1985, 20th ed. p. 44 and 359 The apatite group of minerals commonly exhibit elongated hexagonal prisms. Accretionary prism An accretionary prism or accretionary wedge is formed from sediments that are accreted onto the non-subducting tectonic plate at a convergent plate boundary. Most of the material in the accretionary wedge consists of marine sediments scraped off from the downgoing slab of oceanic crust but in some cases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acicular (crystal Habit)
__NOTOC__ Acicular, in mineralogy, refers to a crystal habit composed of slender, needle-like crystals. Crystals with this habit tend to be fragile. Complete, undamaged acicular specimens are uncommon. The term "acicular" derives from the Late Latin "acicula" meaning "little needle". Strictly speaking, the word refers to a growth habit that is slender and tapering to a point. Prismatic crystals are not acicular; however, colloquial usage has altered the commonly understood meaning of the word. When writing for mineralogical publications, authors should restrict their usage of "acicular" to crystals with the tapering growth habit. To add to the confusion, some minerals are described with various morphological terms. For example, natrolite is often described as slender prismatic and millerite is often described as filiform or capillary. Examples Minerals with an acicular habit include mesolite, natrolite, malachite, gypsum, rutile, brochantite and bultfonteinite. Crystals of dim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Mineralogical Association
Founded in 1958, the International Mineralogical Association (IMA) is an international group of 40 national societies. The goal is to promote the science of mineralogy and to standardize the nomenclature of the 5000 plus known mineral species. The IMA is affiliated with the International Union of Geological Sciences (IUGS). The Association supports the activities of Commissions and Working Groups involved on certain aspects of mineralogical practice and facilitates interactions among mineralogists by sponsoring and organising meetings. In particular, the IMA holds its general meeting every four years. The next meeting is scheduled in 2022 in Lyon, France. Presidents The presidents of the IMA have been: * since 2021: Anhuai Lu ** Peking University *2018–2020: Patrick Cordier (born 1961) ** Université de Lille *2016–2018: Peter C. Burns ** University of Notre Dame *2014–2016: Sergey V. Krivovichev (born 1972) ** Saint Petersburg State University *2012–2014: Walter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
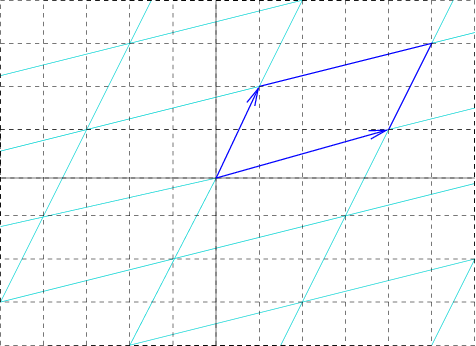
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula Unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. Examples include ionic and and covalent networks such as and C (as diamond or graphite).Steven S. Zumdahl; Susan A. Zumdahl (2000), ''Chemistry'' (5 ed.), Houghton Mifflin, pp. 470-6, Ionic compounds do not exist as individual molecules; a formula unit thus indicates the lowest reduced ratio of ions in the compound. In mineralogy, as minerals are almost exclusively either ionic or network solids, the formula unit is used. The number of formula units (Z) and the dimensions of the crystallographic axes are used in defining the unit cell In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |