|
Efaroxan
Efaroxan is an α2-adrenergic receptor antagonist and antagonist of the imidazoline receptor. Synthesis The Darzens reaction between 2-fluorobenzaldehyde 7848-46-1(1) and Ethyl 2-bromobutyrate 33-68-6(2) gives ethyl 2-ethyl-3-(2-fluorophenyl)oxirane-2-carboxylateCID:100942311(3). A catalytic hydrogenation over Pd/C would give ethyl 2- 2-fluorophenyl)methyl2-hydroxybutanoateCID:77591056(4). Saponification of the ester then gives 2- 2-Fluorophenyl)methyl2-hydroxybutanoic acidCID:53869347(5). Treatment with 2 molar equivalents of sodium hydride apparently gives 2-Ethyl-2,3-dihydrobenzofuran-2-carboxylic acid 11080-50-3(6). Treatment of the carboxylic acid with thionyl chloride then gives the acid chloride and subsequent treatment of this with ethylenediamine in the presence of trimethylaluminium completed the synthesis of ' (8). See also * Fluparoxan * Idazoxan Idazoxan (INN) is a drug which is used in scientific research. It acts as both a selective α2 adrenergic receptor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efaroxan Synthesis
Efaroxan is an α2-adrenergic receptor antagonist and antagonist of the imidazoline receptor. Synthesis The Darzens reaction between 2-fluorobenzaldehyde 7848-46-1(1) and Ethyl 2-bromobutyrate 33-68-6(2) gives ethyl 2-ethyl-3-(2-fluorophenyl)oxirane-2-carboxylateCID:100942311(3). A catalytic hydrogenation over Pd/C would give ethyl 2- 2-fluorophenyl)methyl2-hydroxybutanoateCID:77591056(4). Saponification of the ester then gives 2- 2-Fluorophenyl)methyl2-hydroxybutanoic acidCID:53869347(5). Treatment with 2 molar equivalents of sodium hydride apparently gives 2-Ethyl-2,3-dihydrobenzofuran-2-carboxylic acid 11080-50-3(6). Treatment of the carboxylic acid with thionyl chloride then gives the acid chloride and subsequent treatment of this with ethylenediamine in the presence of trimethylaluminium completed the synthesis of ' (8). See also * Fluparoxan * Idazoxan Idazoxan (INN) is a drug which is used in scientific research. It acts as both a selective α2 adrenergic receptor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-2 Adrenergic Receptor
The alpha-2 (α2) adrenergic receptor (or adrenoceptor) is a G protein-coupled receptor (GPCR) associated with the Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-, α2B-, and α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central and peripheral nervous systems. Cellular localization The α2A adrenergic receptor is localised in the following central nervous system (CNS) structures: * Brainstem (especially the locus coeruleus) * Midbrain * Hypothalamus * Hippocampus * Spinal cord * Cerebral cortex * Cerebellum * Septum Whereas the α2B adrenergic receptor is localised in the following CNS structures: * Olfactory system * Thalamus * Pyramidal layer of the hippocampus * Cerebellar Purkinje layer and the α2C adrenergic receptor is localised in the CN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazoline Receptor
Imidazoline receptors are the primary receptors on which clonidine and other imidazolines act. There are three main classes of imidazoline receptor: I1 is involved in inhibition of the sympathetic nervous system to lower blood pressure, I2 has as yet uncertain functions but is implicated in several psychiatric conditions, and I3 regulates insulin secretion. Classes As of 2017, there are three known subtypes of imidazoline receptors: I1, I2, and I3. I1 receptor The I1 receptor appears to be a G protein-coupled receptor that is localized on the plasma membrane. It may be coupled to PLA2 signalling and thus prostaglandin synthesis. In addition, activation inhibits the sodium-hydrogen antiporter and enzymes of catecholamine synthesis are induced, suggesting that the I1 receptor may belong to the neurocytokine receptor family, since its signaling pathways are similar to those of interleukins. It is found in the neurons of the reticular formation, the dorsomedial medulla oblongata, adr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluparoxan
Fluparoxan (developmental code name GR50360A) is a potent α2-adrenergic receptor antagonist (pKB = 7.9) with excellent selectivity for this receptor over the α1-adrenergic receptor (2,630-fold), and is the only well-studied α2-adrenergic receptor antagonist in its structural family which does not antagonize any variant of the imidazoline receptor. It was shown to possess central α2-adrenoceptor antagonist activity after oral doses in man and was patented as an antidepressant by Glaxo in the early 1980s, but its development was discontinued when the compound failed to show a clear clinical advantage over existing therapies. Pharmacology Pharmacodynamics Fluparoxan is a very selective α2-adrenergic blocker, that readily crosses the blood–brain barrier. Blockade of α2-adrenoreceptors, particularly presynaptic autoreceptors in noradrenergic neurons by fluparoxan, produces an increase in the synaptic concentrations of noradrenaline, by blocking the autoinhibitory feedback me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Idazoxan
Idazoxan (INN) is a drug which is used in scientific research. It acts as both a selective α2 adrenergic receptor antagonist, and an antagonist for the imidazoline receptor. Idazoxan has been under investigation as an antidepressant, but it did not reach the market as such. More recently, it is under investigation as an adjunctive treatment in schizophrenia. Due to its alpha-2 receptor antagonism it is capable of enhancing therapeutic effects of antipsychotics, possibly by enhancing dopamine neurotransmission in the prefrontal cortex of the brain, a brain area thought to be involved in the pathogenesis of schizophrenia. Alzheimer's research Mice treated with idazoxan, which blocks the α2A receptor which regulates norepinephrine, behaved similarly to control animals despite still having amyloid-beta plaques in the brain, as a proof-of-concept experiment that dramatically reduced Alzheimer's pathology and symptoms in two mouse models, potentially offering an immediate treatme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Darzens Reaction
The Darzens reaction (also known as the Darzens condensation or glycidic ester condensation) is the chemical reaction of a ketone or aldehyde with an α- haloester in the presence of a base to form an α,β-epoxy ester, also called a "glycidic ester". This reaction was discovered by the organic chemist Auguste Georges Darzens in 1904. : Reaction mechanism The reaction process begins when a strong base is used to form a carbanion at the halogenated position. Because of the ester, this carbanion is a resonance-stabilized enolate, which makes it relatively easy to form. This nucleophilic structure attacks another carbonyl component, forming a new carbon–carbon bond. These first two steps are similar to a base-catalyzed aldol reaction. The oxygen anion in this aldol-like product then does an intramolecular SN2 attack on the formerly-nucleophilic halide-bearing position, displacing the halide to form an epoxide. This reaction sequence is thus a condensation reaction since there i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. Sodium stearate is a typical soap. Saponification of fats Vegetable oils and animal fats are the traditional materials that are saponified. These greasy materials, triesters called triglycerides, are mixtures derived from diverse fatty acids. Triglycerides can be converted to soap in either a one- or a two-step process. In the traditional one-step process, the triglyceride is treated with a strong base (e.g. lye), which cleaves the ester bond, releasing fatty acid salts (soaps) and glycerol. This process is also the main industrial method for producing glycerol. In some soap-making, the glycerol is left in the soap. If necessary, soaps may be precipitated by salting it out with sodium chloride. Fat in a corpse converts into adipoce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid. Basic properties and structure NaH is produced by the direct reaction of hydrogen and liquid sodium.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride: This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled flask of sulfur dichloride. :SO3 + SCl2 -> SOCl2 + SO2 Other methods includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998.Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, 2005 Wiley-VCH Verlag, Weinheim. Ethylenediamine is the first member of the so-called polyethylene amines. Synthesis Ethylenediamine is produced industrially by treating 1,2-dichloroethane with ammonia under pressure at 180 °C in an aqueous medium:Hans-Jürgen Arpe, Industrielle Organische Chemie, 6. Auflage (2007), Seite 245, Wiley VCH : In this reaction hydrogen chloride is generated, which forms a salt with the amine. The amine is liberated by addition of sodium hydroxide and can then be recovered by . Diethylenetriamine (DETA) and triethylenetetramine (TETA) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
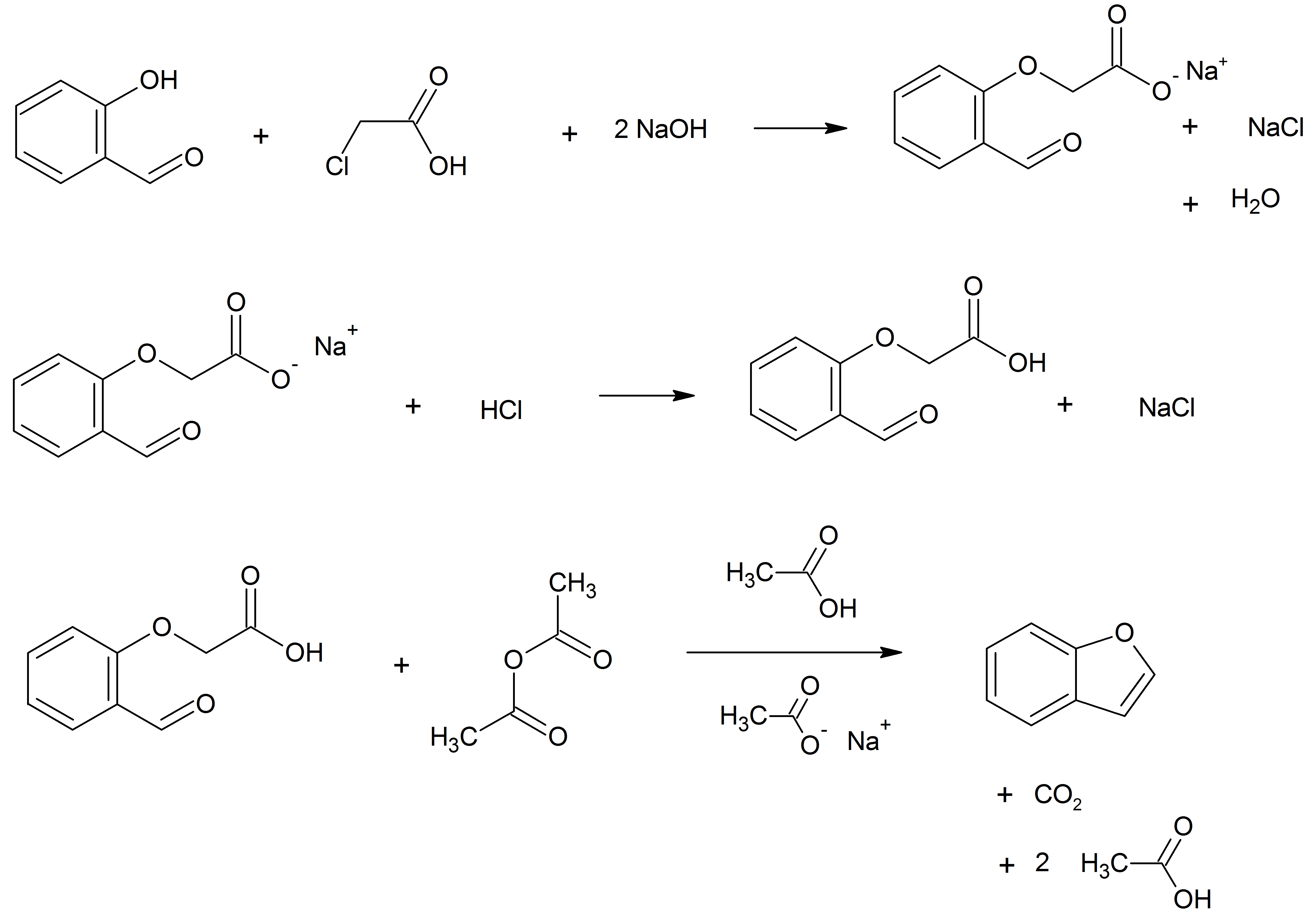
Benzofurans
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |