|
EGFLAM
Pikachurin, also known as AGRINL (AGRINL) and EGF-like, fibronectin type-III and laminin G-like domain-containing protein (EGFLAM), is a protein that in humans is encoded by the ''EGFLAM'' gene. Pikachurin is a dystroglycan-interacting protein which has an essential role in the precise interactions between the photoreceptor cell, photoreceptor ribbon synapse and the bipolar dendrites. The binding with dystroglycan (DG) depends on several factors (glycosylation of DG, presence of divalent cations, presence of other proteins). A non-correct binding between pikachurin and DG is associated with muscular dystrophies that often involve eye abnormalities. Discovery and nomenclature Pikachurin is an extracellular matrix-like retinal protein first discovered in 2008 in Japan by Shigeru Sato et al. and named after Pikachu, a species of the ''Pokémon'' franchise. The name of this protein was inspired by Pikachu's "lightning-fast moves". Pikachurin was initially identified in a microa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pikachurin
Pikachurin, also known as AGRINL (AGRINL) and EGF-like, fibronectin type-III and laminin G-like domain-containing protein (EGFLAM), is a protein that in humans is encoded by the ''EGFLAM'' gene. Pikachurin is a dystroglycan-interacting protein which has an essential role in the precise interactions between the photoreceptor ribbon synapse and the bipolar dendrites. The binding with dystroglycan (DG) depends on several factors ( glycosylation of DG, presence of divalent cations, presence of other proteins). A non-correct binding between pikachurin and DG is associated with muscular dystrophies that often involve eye abnormalities. Discovery and nomenclature Pikachurin is an extracellular matrix-like retinal protein first discovered in 2008 in Japan by Shigeru Sato et al. and named after Pikachu, a species of the '' Pokémon'' franchise. The name of this protein was inspired by Pikachu's "lightning-fast moves". Pikachurin was initially identified in a microarray analysis of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
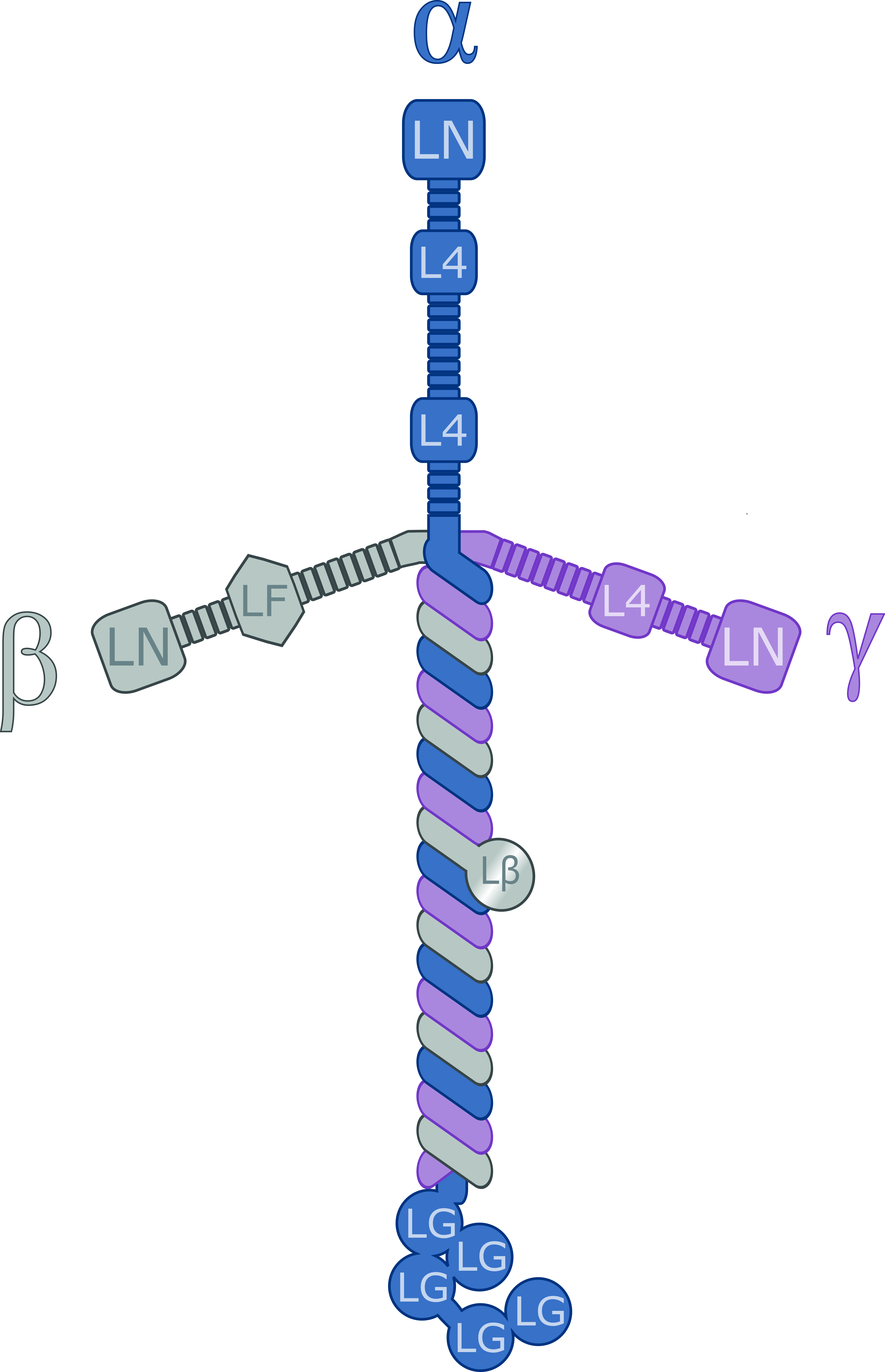
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecules, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystroglycan
Dystroglycan is a protein that in humans is encoded by the ''DAG1'' gene. Dystroglycan is one of the dystrophin-associated glycoproteins, which is encoded by a 5.5 kb transcript in ''Homo sapiens'' on chromosome 3. There are two exons that are separated by a large intron. The spliced exons code for a protein product that is finally cleaved into two non-covalently associated subunits, lpha(N-terminal) and eta(C-terminal). Function In skeletal muscle the dystroglycan complex works as a transmembrane linkage between the extracellular matrix and the cytoskeleton. lphadystroglycan is extracellular and binds to merosin lpha2 laminin in the basement membrane, while etadystroglycan is a transmembrane protein and binds to dystrophin, which is a large rod-like cytoskeletal protein, absent in Duchenne muscular dystrophy patients. Dystrophin binds to intracellular actin cables. In this way, the dystroglycan complex, which links the extracellular matrix to the intracellular actin cab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and diploblasts. It is a structure composed of nervous tissue positioned along the rostral (nose end) to caudal (tail end) axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain (precursor structures exist in onychophorans, gastropods and lancelets). The rest of this article exclusively discusses the vertebrate central nervous system, which is radically distinct from all other animals. Overview In vertebrates, the brain and spinal cord are both enclosed in the meninges. The meninges provide a barrier to chemicals dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synapse
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell. Synapses are essential to the transmission of nervous impulses from one neuron to another. Neurons are specialized to pass signals to individual target cells, and synapses are the means by which they do so. At a synapse, the plasma membrane of the signal-passing neuron (the ''presynaptic'' neuron) comes into close apposition with the membrane of the target (''postsynaptic'') cell. Both the presynaptic and postsynaptic sites contain extensive arrays of molecular machinery that link the two membranes together and carry out the signaling process. In many synapses, the presynaptic part is located on an axon and the postsynaptic part is located on a dendrite or soma. Astrocytes also exchange information with the synaptic neurons, responding to synaptic activity and, in turn, regulating neurotransmission. Syna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribbon Synapse
The ribbon synapse is a type of neuronal synapse characterized by the presence of an electron-dense structure, the synaptic ribbon, that holds vesicles close to the active zone. It is characterized by a tight vesicle-calcium channel coupling that promotes rapid neurotransmitter release and sustained signal transmission. Ribbon synapses undergo a cycle of exocytosis and endocytosis in response to graded changes of membrane potential. It has been proposed that most ribbon synapses undergo a special type of exocytosis based on coordinated multivesicular release. This interpretation has recently been questioned at the inner hair cell ribbon synapse, where it has been instead proposed that exocytosis is described by uniquantal (i.e., univesicular) release shaped by a flickering vesicle fusion pore. These unique features specialize the ribbon synapse to enable extremely fast, precise and sustained neurotransmission, which is critical for the perception of complex senses such as vision and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nestin (protein)
Nestin is a protein that in humans is encoded by the NES gene. Nestin (acronym for neuroepithelial stem cell protein) is a type VI intermediate filament (IF) protein. These intermediate filament proteins are expressed mostly in nerve cells where they are implicated in the radial growth of the axon. Seven genes encode for the heavy (NF-H), medium (NF-M) and light neurofilament (NF-L) proteins, nestin and α-internexin in nerve cells, synemin α and desmuslin/synemin β (two alternative transcripts of the DMN gene) in muscle cells, and syncoilin (also in muscle cells). Members of this group mostly preferentially coassemble as heteropolymers in tissues. Steinert et al. has shown that nestin forms homodimers and homotetramers but does not form IF by itself in vitro. In mixtures, nestin preferentially co-assembles with purified vimentin or the type IV IF protein internexin to form heterodimer coiled-coil molecules. Gene Structurally, nestin has the shortest head domain (N-terminus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroretinogram
Electroretinography measures the electrical responses of various cell types in the retina, including the photoreceptors ( rods and cones), inner retinal cells ( bipolar and amacrine cells), and the ganglion cells. Electrodes are placed on the surface of the cornea (DTL silver/nylon fiber string or ERG jet) or on the skin beneath the eye (sensor strips) to measure retinal responses. Retinal pigment epithelium (RPE) responses are measured with an EOG test with skin-contact electrodes placed near the canthi. During a recording, the patient's eyes are exposed to standardized stimuli and the resulting signal is displayed showing the time course of the signal's amplitude (voltage). Signals are very small, and typically are measured in microvolts or nanovolts. The ERG is composed of electrical potentials contributed by different cell types within the retina, and the stimulus conditions (flash or pattern stimulus, whether a background light is present, and the colors of the stimulus a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurexin
Neurexins (NRXN) are a family of presynaptic cell adhesion proteins that have roles in connecting neurons at the synapse. They are located mostly on the presynaptic membrane and contain a single transmembrane domain. The extracellular domain interacts with proteins in the synaptic cleft, most notably neuroligin, while the intracellular cytoplasmic portion interacts with proteins associated with exocytosis. Neurexin and neuroligin "shake hands," resulting in the connection between the two neurons and the production of a synapse. Neurexins mediate signaling across the synapse, and influence the properties of neural networks by synapse specificity. Neurexins were discovered as receptors for α-latrotoxin, a vertebrate-specific toxin in black widow spider venom that binds to presynaptic receptors and induces massive neurotransmitter release. In humans, alterations in genes encoding neurexins are implicated in autism and other cognitive diseases, such as Tourette syndrome and schiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agrin
Agrin is a large proteoglycan whose best-characterised role is in the development of the neuromuscular junction during embryogenesis. Agrin is named based on its involvement in the aggregation of acetylcholine receptors during synaptogenesis. In humans, this protein is encoded by the ''AGRN'' gene. This protein has nine domains homologous to protease inhibitors. It may also have functions in other tissues and during other stages of development. It is a major proteoglycan component in the glomerular basement membrane and may play a role in the renal filtration and cell-matrix interactions. Agrin functions by activating the MuSK protein (for Muscle-Specific Kinase), which is a receptor tyrosine kinase required for the formation and maintenance of the neuromuscular junction. Agrin is required to activate MuSK, which is similarly also required for neuromuscular junction formation. Discovery Agrin was first identified by the U.J. McMahan laboratory, Stanford University. Mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perlecan
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the ''HSPG2'' gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. Perlecan was originally isolated from a tumor cell line and shown to be present in all native basement membranes. Perlecan is a large multidomain (five domains, labeled I-V) proteoglycan that binds to and cross-links many extracellular matrix (ECM) components and cell-surface molecules. Perlecan is synthesized by both vascular endothelial and smooth muscle cells and deposited in the extracellular matrix of parahoxozoans. Perlecan is highly conserved across species and the available data indicate that it has evolved from ancient ancestors by gene duplication and exon shuffling. Structure Perlecan consists of a core protein of molecular weight 4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |