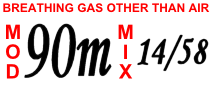|
Equivalent Narcotic Depth
Equivalent narcotic depth (END) is used in technical diving as a way of estimating the narcotic effect of a breathing gas mixture, such as heliox and trimix. The method is, for a given mix and depth, to calculate the depth which would produce the same narcotic effect as when breathing air. The equivalent narcotic depth of a breathing gas mix at a particular depth is calculated by finding the depth of a dive when breathing air that would have the same total partial pressure of nitrogen and oxygen Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ... as the breathing gas in question. For example, a trimix containing 20% oxygen, 40% helium, 40% nitrogen (trimix 20/40) being used at has an END of . Since air is composed of approximately 21% oxygen and 79% nitrogen, the narcotic gase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technical Diving
Technical diving (also referred to as tec diving or tech diving) is scuba diving that exceeds the agency-specified limits of recreational diving for non-professional purposes. Technical diving may expose the diver to hazards beyond those normally associated with recreational diving, and to a greater risk of serious injury or death. The risk may be reduced by appropriate skills, knowledge and experience, and by using suitable equipment and procedures. The skills may be developed through appropriate specialised training and experience. The equipment often involves breathing gases other than air or standard nitrox mixtures, and multiple gas sources. The popularisation of the term ''technical diving'' has been credited to Michael Menduno, who was editor of the (now defunct) diving magazine ''aquaCorps Journal'', but the concept and term, ''technical diving'', go back at least as far as 1977,In his 1989 book, ''Advanced Wreck Diving'', author and leading technical diver, Gary Gentile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Narcosis
Narcosis while diving (also known as nitrogen narcosis, inert gas narcosis, raptures of the deep, Martini effect) is a reversible alteration in consciousness that occurs while diving at depth. It is caused by the anesthetic effect of certain gases at high pressure. The Greek word (narkōsis), "the act of making numb", is derived from (narkē), "numbness, torpor", a term used by Homer and Hippocrates. Narcosis produces a state similar to drunkenness (alcohol intoxication), or nitrous oxide inhalation. It can occur during shallow dives, but does not usually become noticeable at depths less than . Except for helium and probably neon, all gases that can be breathed have a narcotic effect, although widely varying in degree. The effect is consistently greater for gases with a higher lipid solubility, and although the mechanism of this phenomenon is still not fully clear, there is good evidence that the two properties are mechanistically related. As depth increases, the men ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breathing Gas
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed habitats such as scuba equipment, surface supplied diving equipment, recompression chambers, high-altitude mountaineering, high-flying aircraft, submarines, space suits, spacecraft, medical life support and first aid equipment, and anaesthetic machines. Oxygen is the essential component for any breathing gas, at a partial pressure of between roughly 0.16 and 1.60 bar at the ambient pressure. The oxygen is usually the only metabolically active component unless the gas is an anaesthetic mixture. Some of the oxygen in the breathing gas is consumed by the metabolic processes, and the inert components are unchanged, and serve mainly to dilute the oxygen to an appropriate concentration, and are therefore also known as diluent gases. Most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heliox
Heliox is a breathing gas mixture of helium (He) and oxygen (O2). It is used as a medical treatment for patients with difficulty breathing because mixture generates less resistance than atmospheric air when passing through the airways of the lungs, and thus requires less effort by a patient to breathe in and out of the lungs. It is also used as a breathing gas diluent for deep ambient pressure diving as it is not narcotic at high pressure, and for its low work of breathing. Heliox has been used medically since the 1930s, and although the medical community adopted it initially to alleviate symptoms of upper airway obstruction, its range of medical uses has since expanded greatly, mostly because of the low density of the gas. Heliox is also used in saturation diving and sometimes during the deep phase of technical dives. Medical uses In medicine heliox may refer to a mixture of 21% O2 (the same as air) and 79% He, although other combinations are available (70/30 and 60/40). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimix (breathing Gas)
Trimix is a breathing gas consisting of oxygen, helium and nitrogen and is used in deep commercial diving, during the deep phase of dives carried out using technical diving techniques, and in advanced recreational diving. The helium is included as a substitute for some of the nitrogen, to reduce the narcotic effect of the breathing gas at depth. With a mixture of three gases it is possible to create mixes suitable for different depths or purposes by adjusting the proportions of each gas. Oxygen content can be optimised for the depth to limit the risk of toxicity, and the inert component balanced between nitrogen (which is cheap but narcotic) and helium (which is not narcotic and reduces work of breathing, but is more expensive and increases heat loss). The mixture of helium and oxygen with a 0% nitrogen content is generally known as heliox. This is frequently used as a breathing gas in deep commercial diving operations, where it is often recycled to save the expensive heli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure
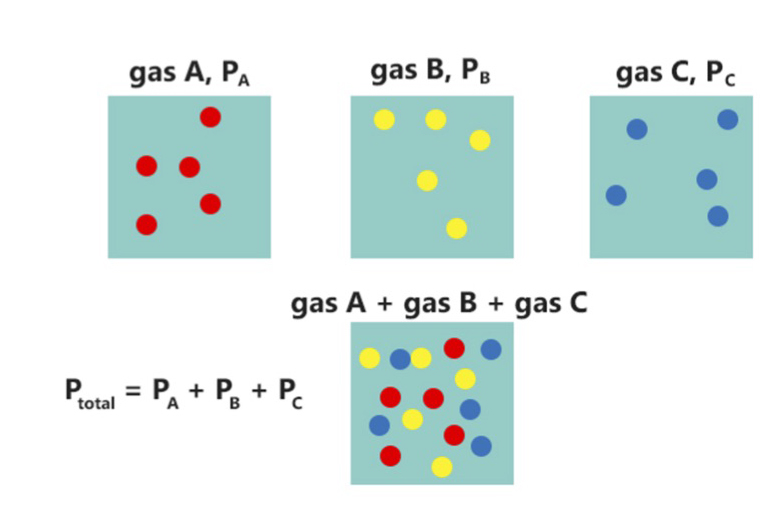
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outdated Theories Of Anaesthetic Action
A general anaesthetic (or anesthetic) is a drug that brings about a reversible loss of consciousness. These drugs are generally administered by an anaesthetist/anesthesiologist in order to induce or maintain general anaesthesia to facilitate surgery. General anaesthetics have been widely used in surgery since 1842 when Crawford Long for the first time administered diethyl ether to a patient and performed a painless operation. It has long been believed that general anaesthetics exert their effects (analgesia, unconsciousness, immobility) through a membrane mediated mechanism or by directly modulating the activity of membrane proteins in the neuronal membrane. In general, different anaesthetics exhibit different mechanisms of action such that there are numerous molecular targets at all levels of integration within the central nervous system. However, for certain intravenous anaesthetics, such as propofol and etomidate, the main molecular target has been identified to be GABAA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |