|
Enterobacter Gergoviae
''Pluralibacter gergoviae'' (formerly ''Enterobacter gergoviae'') is a Gram-negative, motile, facultatively-anaerobic, rod-shaped bacterium. ''P. gergoviae'' is of special interest to the cosmetics industry, as it displays resistance to parabens, a common antimicrobial agent added to cosmetic products. Background ''Enterobacter gergoviae'' was first proposed as a novel species in 1980. The species name is derived from the Gergovie plateau, which is located near Clermont-Ferrand University Hospital; the type strain was isolated at this hospital during a nosocomial outbreak of ''P. gergoviae.'' In 2013, the species was reclassified into the novel genus, ''Pluralibacter'', and is the type species for the genus. ''Pluralibacter gergoviae'' has been isolated from maize, grapes, coffee beans, spring water, fruit flies, and pink bollworms. It is an uncommon human pathogen, most commonly as an opportunistic nosocomial infection. One hospital in Spain reported the organism to represent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism ''Escherichia coli'', as well as many pathogenic bacteria, such as ''Pseudomonas aeruginosa'', ''Chlamydia trachomatis'', and ''Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a complex lipo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxacillin
Oxacillin (trade name Bactocill) is a narrow-spectrum beta-lactam antibiotic of the penicillin class developed by Beecham. It was patented in 1960 and approved for medical use in 1962. Medical uses Oxacillin is a penicillinase-resistant ╬▓-lactam. It is similar to methicillin, and has replaced methicillin in clinical use. Other related compounds are nafcillin, cloxacillin, dicloxacillin, and flucloxacillin. Since it is resistant to penicillinase enzymes, such as that produced by ''Staphylococcus aureus'', it is widely used clinically in the US to treat penicillin-resistant ''Staphylococcus aureus''. However, with the introduction and widespread use of both oxacillin and methicillin, antibiotic-resistant strains called methicillin-resistant and oxacillin-resistant ''Staphylococcus aureus'' (MRSA/ORSA) have become increasingly prevalent worldwide. MRSA/ORSA can be treated with vancomycin or other new antibiotics. Contraindications The use of oxacillin is contraindicated in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
╬▓-lactamase
Beta-lactamases, (╬▓-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems ( ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (╬▓-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the ╬▓-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are typically used to target a broad spectrum of gram-positive and gram-negative bacteria. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a ''Streptomyces'' serine ╬▓-lactamase (SBLs) is given by . The alpha-beta fold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cefoxitin
Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium. History and discovery Groups of researchers at Merck and Lilly discovered Cephamycin C while looking at penicillin-producing bacteria. This followed their discovery of erythromycin, another antibiotic. Cephamycin C was the first cephem discovered but while it was highly resistant to several beta-lactamases, as is its derivative cefoxitin, it was almost only effective against Gram negative bacteria. The scientists used chemically modified the compound to give cefoxitin, so titled due to its semi-synthetic nature. This new mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fosfomycin
Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat lower UTI. It is not indicated for kidney infections. Occasionally it is used for prostate infections. It is generally taken by mouth. Common side effects include diarrhea, nausea, headache, and vaginal yeast infections. Severe side effects may include anaphylaxis and ''Clostridium difficile''-associated diarrhea. While use during pregnancy has not been found to be harmful, such use is not recommended. A single dose when breastfeeding appears safe. Fosfomycin works by interfering with the production of the bacterial cell wall. Fosfomycin was discovered in 1969 and approved for medical use in the United States in 1996 . It is on the World Health Organization's List of Essential Medicines. The World Health Organization classifies fosfomycin as critically important for human medicine. It is available as a generic medication. It was originally produced by certain types of ''Stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rifampicin
Rifampicin, also known as rifampin, is an ansamycin antibiotic used to treat several types of bacterial infections, including tuberculosis (TB), mycobacterium avium complex, ''Mycobacterium avium'' complex, leprosy, and LegionnairesÔÇÖ disease. It is almost always used together with other antibiotics with two notable exceptions: when given as a "preferred treatment that is strongly recommended" for latent TB infection; and when used as post-exposure prophylaxis to prevent Haemophilus influenzae type b, ''Haemophilus influenzae'' type b and meningococcal disease in people who have been exposed to those bacteria. Before treating a person for a long period of time, measurements of liver enzymes and blood counts are recommended. Rifampicin may be given either Oral administration, by mouth or intravenously. Common side effects include nausea, vomiting, diarrhea, and loss of appetite. It often turns urine, sweat, and tears a red or orange color. Liver problems or allergic reactions ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptogramins
Streptogramins are a class of antibiotics. Streptogramins are effective in the treatment of vancomycin-resistant ''Staphylococcus aureus'' (VRSA) and vancomycin-resistant ''Enterococcus'' (VRE), two of the most rapidly growing strains of multidrug-resistant bacteria. They fall into two groups: streptogramin A and streptogramin B. Members include: * Quinupristin/dalfopristin * Pristinamycin * Virginiamycin * NXL 103 Linopristin/flopristin (development codes NXL103 and XRP 2868) is an experimental drug candidate under development by Novexel. It is an oral streptogramin antibiotic that has potent ''in vitro'' activity against certain Gram-positive bacteria i ..., an experimental streptogramin in clinical trials for the treatment of respiratory tract infections. References Antibiotics {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clindamycin
Clindamycin is an antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media (middle ear infections), and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant ''Staphylococcus aureus'' (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina. Common side effects include nausea and vomiting, diarrhea, rashes, and pain at the site of injection. It increases the risk of hospital-acquired ''Clostridium difficile'' colitis about fourfold and thus is only recommended when other antibiotics are not appropriate. Alternative antibiotics may be recommended as a result. It appears to be generally safe in pregnancy. It is of the lincosamide class and works by blocking bacter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincomycin
Lincomycin is a lincosamide antibiotic that comes from the actinomycete ''Streptomyces lincolnensis''. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964. Uses Although similar in antibacterial spectrum and mechanism of action to macrolides, lincomycin is also effective against other organisms including actinomycetes and some species of '' Mycoplasma'' and ''Plasmodium''. However, because of its adverse effects and toxicity, it is rarely used today and reserved for patients allergic to penicillin or where bacteria have developed resistance. Clinical pharmacology Intramuscular administration of a single dose of 600 mg of Lincomycin produces average peak serum levels of 11.6 ┬Ág/mL at 60 min, and maintains therapeutic levels for 17 h to 20 h, for most susceptible gram-positive organis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincosamides
Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin. Structure Lincosamides consist of a pyrrolidine ring linked to a pyranose moiety (methylthio-lincosamide) via an amide bond. Hydrolysis of lincosamides, specifically lincomycin, splits the molecule into its building blocks of the sugar and proline moieties. Both of these derivatives can conversely be recombined into the drug itself or a derivative. Synthesis Biosynthesis of lincosamides occurs through a biphasic pathway, in which propylproline and methylthiolincosamide are independently synthesized immediately before condensation of the two precursor molecules. Condensation of the propylproline carboxyl group with the methylthiolincosamide amine group via an amide bond forms ''N''-demethyllincomycin. ''N''-Demethyllincomycin is subsequently methylated via ''S''-adenosyl methionine to produce lincomycin A. Lincomycin is naturally produced by bacteria species, namely ''Streptomy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
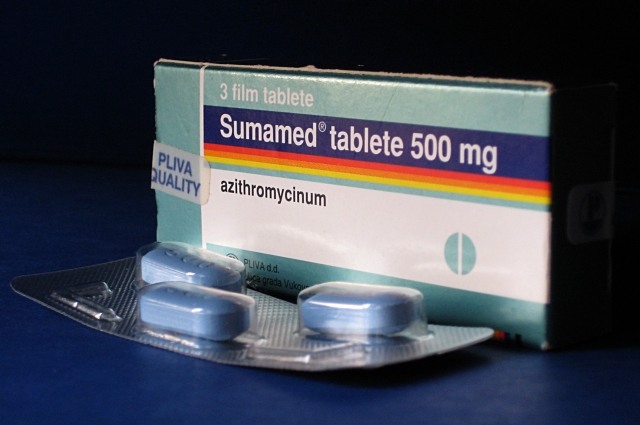
Azithromycin
Azithromycin, sold under the brand names Zithromax (in oral form) and Azasite (as an eye drop), is an antibiotic medication used for the treatment of a number of bacterial infections. This includes middle ear infections, strep throat, pneumonia, traveler's diarrhea, and certain other intestinal infections. Along with other medications, it may also be used for malaria. It can be taken by mouth or intravenously. Common side effects include nausea, vomiting, diarrhea and upset stomach. An allergic reaction, such as anaphylaxis, QT prolongation, or a type of diarrhea caused by ''Clostridium difficile'' is possible. No harm has been found with its use during pregnancy. Its safety during breastfeeding is not confirmed, but it is likely safe. Azithromycin is an azalide, a type of macrolide antibiotic. It works by decreasing the production of protein, thereby stopping bacterial growth. Azithromycin was discovered in 1980 by the Yugoslav pharmaceutical company Pliva and approved f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |