|
Doxacurium
Doxacurium chloride (formerly recognized as BW938U80 or BW A938U) is a neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to provide skeletal muscle relaxation during surgery or mechanical ventilation. Unlike a number of other related skeletal muscle relaxants, it is rarely used adjunctively to facilitate endotracheal intubation. Chemistry Doxacurium is a symmetrical molecule because it is a diester of succinic acid. The pharmacological action of doxacurium is a function of its competitive antagonism to acetylcholine receptors of the nicotinic type. The drug is marketed worldwide under the tradename of Nuromax, and it is classified as a long-duration non-depolarizing neuromuscular blocking agent in a class of compounds commonly and most erroneously referred to as "benzylisoquinolines" when, in fact, it is a ''bis''benzyltetrahydroisoquinolinium agent. The pharmaceutical prepar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
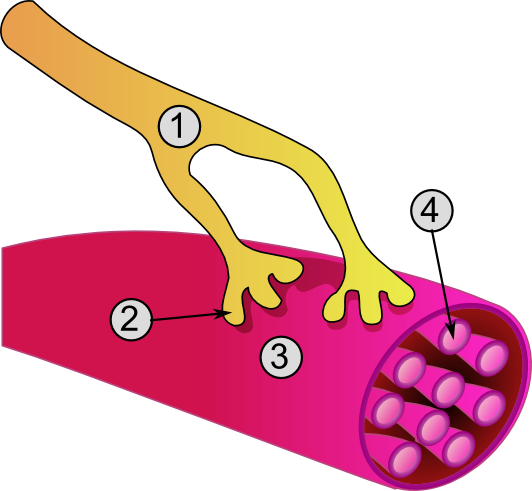
Neuromuscular-blocking Drugs
Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors. In clinical use, neuromuscular block is used adjunctively to anesthesia to produce paralysis, firstly to paralyze the vocal cords, and permit intubation of the trachea, and secondly to optimize the surgical field by inhibiting spontaneous ventilation, and causing relaxation of skeletal muscles. Because the appropriate dose of neuromuscular-blocking drug may paralyze muscles required for breathing (i.e., the diaphragm), mechanical ventilation should be available to maintain adequate respiration. Patients are still aware of pain even after full conduction block has occurred; hence, general anesthetics and/or analgesics must also be given to prevent anesthesia awareness. Nomenclature Neuromuscular blocking drugs are often classified into two broad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mivacurium Chloride
Mivacurium chloride (formerly recognized as BW1090U81, BW B1090U or BW1090U) is a short-duration non-depolarizing neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Structure Mivacurium is a symmetrical molecule existing as a mixture of three of twenty possible isomers: the isomerism stems from chirality at the C-1 carbon position of both the tetrahydroisoquinolinium rings, as well as both the positively charged nitrogen (onium) heads, and the E/Z diastereomerism at the C=C double bond of the oct-4-ene diester bridge. Thus, owing to the symmetry and chirality, the three isomers of mivacurium are (E)-1R,1'R,2R,2'R, (identified as BW1217U84), (E)-1R,1'R,2R,2'S, (BW1333U83) and (E)-1R,1'R,1'S,2'S, (BW1309U83). These are also known as ''cis''-''cis'', ''cis''-'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mivacurium
Mivacurium chloride (formerly recognized as BW1090U81, BW B1090U or BW1090U) is a short-duration non-depolarizing neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Structure Mivacurium is a symmetrical molecule existing as a mixture of three of twenty possible isomers: the isomerism stems from chirality at the C-1 carbon position of both the tetrahydroisoquinolinium rings, as well as both the positively charged nitrogen (onium) heads, and the E/Z diastereomerism at the C=C double bond of the oct-4-ene diester bridge. Thus, owing to the symmetry and chirality, the three isomers of mivacurium are (E)-1R,1'R,2R,2'R, (identified as BW1217U84), (E)-1R,1'R,2R,2'S, (BW1333U83) and (E)-1R,1'R,1'S,2'S, (BW1309U83). These are also known as ''cis''-''cis'', ''cis''-' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuromuscular Blocking Drugs
Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors. In clinical use, neuromuscular block is used adjunctively to anesthesia to produce paralysis, firstly to paralyze the vocal cords, and permit intubation of the trachea, and secondly to optimize the surgical field by inhibiting spontaneous ventilation, and causing relaxation of skeletal muscles. Because the appropriate dose of neuromuscular-blocking drug may paralyze muscles required for breathing (i.e., the diaphragm), mechanical ventilation should be available to maintain adequate respiration. Patients are still aware of pain even after full conduction block has occurred; hence, general anesthetics and/or analgesics must also be given to prevent anesthesia awareness. Nomenclature Neuromuscular blocking drugs are often classified into two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laudexium
Laudexium metilsulfate is a neuromuscular blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in surgical anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Laudexium is no longer used in clinical practice, though it was introduced clinically in the early 1950s. It has about half the potency, a slower onset of action and a duration of action much longer than that of ''d''-tubocurarine Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or .... As with all clinically established (as well as experimental agents) with a non-depolarizing mechanism of action, its pharmacological action can be antagonized by anticholinesterases. The displace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrogallol Ethers
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a water-soluble, white solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomers of benzenetriols. Production and reactions It is produced in the manner first reported by Scheele in 1786: heating gallic acid to induce decarboxylation. Gallic acid is also obtained from tannin. Many alternative routes have been devised. One preparation involves treating ''para''-chlorophenoldisulfonic acid with potassium hydroxide, a variant on the time-honored route to phenols from sulfonic acids. When in alkaline solution, pyrogallol undergoes deprotonation of one or more phenolic groups. Such solutions absorb oxygen from the air, turning brown. This conversion can be used to determine the amount of oxygen in a gas sample, notably by the use of the Orsat apparatus. Polyhydroxybenzenes are relatively electron-rich. One manifestation is the easy C-acetylation of pyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norsalsolinol Ethers
Norsalsolinol is a chemical compound that is produced naturally in the body through metabolism of dopamine. It has been shown to be a selective dopaminergic neurotoxin, and has been suggested as a possible cause of neurodegenerative conditions such as Parkinson's disease and the brain damage associated with alcoholism, although evidence for a causal relationship is unclear. (R)-Salsolinol which has been shown to be a product of ethanol metabolism, stereospecifically induces behavioral sensitization and leads to excessive alcohol intake in rats See also * 6-Hydroxydopamine * MPTP * Rotenone Rotenone is an odorless, colorless, crystalline isoflavone used as a broad-spectrum insecticide, piscicide, and pesticide. It occurs naturally in the seeds and stems of several plants, such as the jicama vine plant, and the roots of several member ... References Tetrahydroisoquinolines Catechols {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Antagonists
A nicotinic antagonist is a type of anticholinergic drug that inhibits the action of acetylcholine (ACh) at nicotinic acetylcholine receptors. These compounds are mainly used for peripheral muscle paralysis in surgery, the classical agent of this type being tubocurarine,P. Taylor (1990). In ''Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th Ed.'', (A. G. Gilman et al., Eds.), pp. 166-186, New York: Pergamon Press. but some centrally acting compounds such as bupropion, mecamylamine, and 18-methoxycoronaridine block nicotinic acetylcholine receptors in the brain and have been proposed for treating nicotine addiction. *Note: Succinylcholine is a nicotinic agonist. See neuromuscular blocking agents page for details on the mechanism of action. See also * Nicotinic acetylcholine receptor * Nicotinic agonist * Muscarinic acetylcholine receptor * Muscarinic agonist * Muscarinic antagonist A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscle Relaxants
A muscle relaxant is a drug that affects skeletal muscle function and decreases the muscle tone. It may be used to alleviate symptoms such as muscle spasms, pain, and hyperreflexia. The term "muscle relaxant" is used to refer to two major therapeutic groups: neuromuscular blockers and spasmolytics. Neuromuscular blockers act by interfering with transmission at the neuromuscular end plate and have no central nervous system (CNS) activity. They are often used during surgical procedures and in intensive care and emergency medicine to cause temporary paralysis. Spasmolytics, also known as "centrally acting" muscle relaxant, are used to alleviate musculoskeletal pain and spasms and to reduce spasticity in a variety of neurological conditions. While both neuromuscular blockers and spasmolytics are often grouped together as muscle relaxant, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Compounds
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammonium compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decamethonium
Decamethonium (Syncurine) is a depolarizing muscle relaxant or neuromuscular blocking agent, and is used in anesthesia to induce paralysis. Pharmacology Decamethonium, which has a short action time, is similar to acetylcholine and acts as a partial agonist of the nicotinic acetylcholine receptor. In the motor endplate, it causes depolarization, preventing further effects to the normal release of acetylcholine from the presynaptic terminal, and therefore preventing the neural stimulus from affecting the muscle. In the process of binding, decamethonium activates (depolarizes) the motor endplate - but since the decamethonium itself is not degraded, the membrane remains depolarized and unresponsive to normal acetylcholine release. Contraindications/limitations Decamethonium does not produce unconsciousness or anesthesia, and its effects may cause considerable psychological distress while simultaneously making it impossible for a patient to communicate. For these reasons, admi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |