|
Diterpene
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abietane
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes. Abietanes are found in the tissues and resins of certain higher plants, particularly gymnosperms. Although the functions of terpenes are not fully understood, conifers appear to produce abietane diterpenoids as a form of defense against insect and microbial attack. Some abietane diterpenoids, especially aromatic abietenes, are of interest to the pharmacology and natural products communities for their potential biological activities. In the rock record, abietanes are commonly found in amber as well as in fossil wood, sometimes in the form of the mineral fichtelite. Additionally, abietanes are observed in sediments—both riverine and marine—and in coals, where they are often interpreted as geochemical biomarkers for terrestrial input from conifers. Chemical str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stemarene
Stemarene is a diterpene hydrocarbon can be produced biosynthetically through enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ... extracts from rice. References Diterpenes Cyclopentanes {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taxadiene
Taxadiene (taxa-4,11-diene) is a diterpene. Taxadiene is the first committed intermediate in the synthesis of taxol. Six hydroxylation reactions, and a few others, are needed to convert taxadiene to baccatin III. Enzymatically, taxadiene is produced from geranylgeranyl pyrophosphate by taxadiene synthase. A biochemical gram-scale production of taxadiene has been reported in 2010 using genetically engineered ''Escherichia coli ''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...''. References Dienes Taxanes Diterpenes {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sclarene
Sclarene is a diterpene present in the foliage of ''Podocarpus hallii ''Podocarpus laetus'' is a species of conifer in the family Podocarpaceae, commonly known as Hall's tōtara, mountain tōtara or thin-barked tōtara. Previously known as ''Podocarpus hallii'' and ''Podocarpus cunninghamii'', in 2015 it was re ...''. References Diterpenes Decalins Polyenes Vinylidene compounds {{organic-compound-stub ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
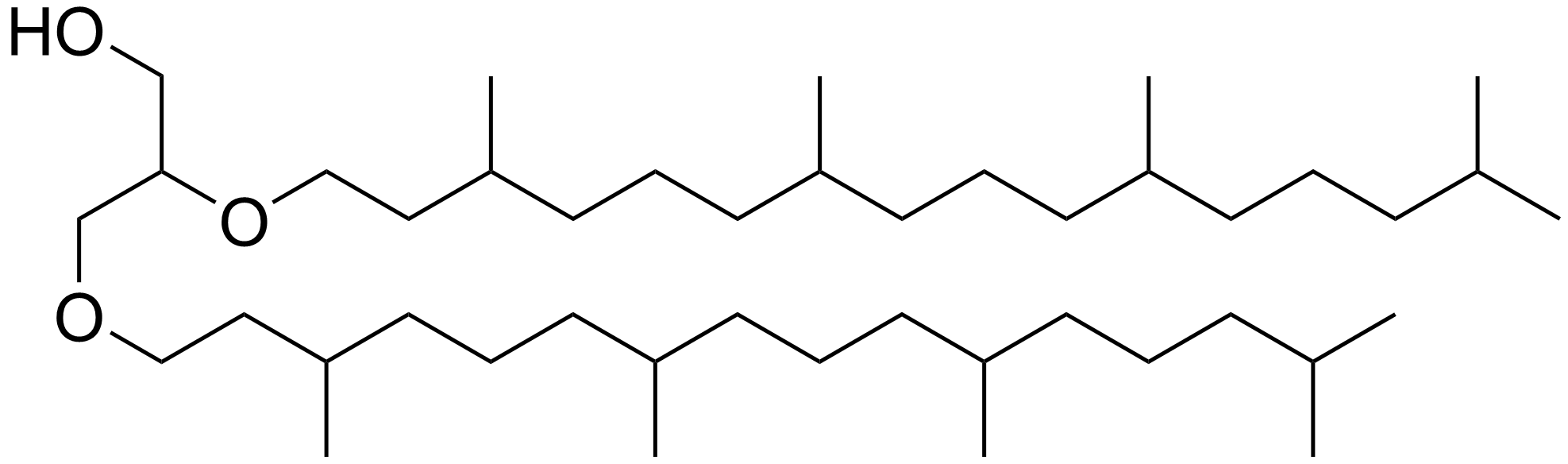
Phytol
Phytol (florasol, phytosol) is an acyclic hydrogenated diterpene alcohol that can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1. In ruminants, the gut fermentation of ingested plant materials liberates phytol, a constituent of chlorophyll, which is then converted to phytanic acid and stored in fats. In shark liver it yields pristane. Human pathology Refsum disease (also known as adult Refsum disease) is an autosomal recessive disorder that results in the accumulation of toxic stores of phytanic acid in tissues and frequently manifests as a variable combination of peripheral polyneuropathy, cerebellar ataxia, retinitis pigmentosa, anosmia, and hearing loss. Although humans cannot derive phytanic acid from chlorophyll, they can convert free phytol into phytanic acid. Thus, patients with Refsum disease should limit their intake of phytanic acid and free phytol. The amount of free phytol in numerous food products has been reported. Roles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinol
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family found in food and used as a dietary supplement. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xerophthalmia. In regions where deficiency is common, a single large dose is recommended to those at high risk twice a year. It is also used to reduce the risk of complications in measles patients. It is taken by mouth or by injection into a muscle. Retinol at normal doses is well tolerated. High doses may cause enlargement of the liver, dry skin, and hypervitaminosis A. High doses during pregnancy may harm the fetus. The body converts retinol to retinal and retinoic acid, through which it acts. Dietary sources include fish, dairy products, and meat. Retinol was discovered in 1909, isolated in 1931, and first made in 1947. It is on the World Health Organization's List of Essential Medicines. Retinol is available as a generic medication and over ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phylloquinone
Phytomenadione, also known as vitamin K1 or phylloquinone, is a vitamin found in food and used as a dietary supplement. It is on the World Health Organization's List of Essential Medicines. As a supplement it is used to treat certain bleeding disorders. This includes warfarin overdose, vitamin K deficiency, and obstructive jaundice. It is also recommended to prevent and treat vitamin K deficiency bleeding in infants. Use is typically recommended by mouth, intramuscular injection or injection under the skin. When given by injection benefits are seen within two hours. Many countries in the world choose intramuscular injections in newborn to keep them safe from severe bleeding (VKDB). It is considered a safe treatment and saves many children from death and severe neurologic deficit every year. Side effects when given by injection may include pain at the site of injection. Severe allergic reactions may occur when it is injected into a vein or muscle, but this has mainly happened ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytyl
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytane
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stemodene
Stemodene is a labdane-related diterpene whose corresponding terpene synthase has been discovered in rice and subsequently cloned and functionally characterized. The gene responsible for stemodene production has not been found in the completed rice genome, thus suggesting that perhaps other genes are as yet undiscovered in the "completed" genome. Stemarene synthase demonstrates high sequence homology with stemodene synthase, thus accounting for the latter's discovery by Dana Morrone in 2005. Additionally, the corresponding olefin produced by each cyclase A cyclase is an enzyme, almost always a lyase, that catalyzes a chemical reaction to form a cyclic compound. Important cyclase enzymes include: * Adenylyl cyclase, which forms cyclic AMP from adenosine triphosphate (EC 4.6.1.1) ** ADCY1 ** ADCY2 ... shows structural similarities and is derived from the common precursor of ''syn''-copalyl diphosphate. References Diterpenes Cyclopentanes {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Labdane
Labdane is a natural bicyclic diterpene. It forms the structural core for a wide variety of natural products collectively known as ''labdanes'' or ''labdane diterpenes''. The labdanes were so named because the first members of the class were originally obtained from labdanum, a resin derived from the gum rockrose. A variety of biological activities have been determined for labdane diterpenes including antibacterial, antifungal, antiprotozoal, and anti-inflammatory activities. Example labdane derivatives * Forskolin * Galanolactone * Isocupressic acid - is an abortifacient component of ''Cupressus macrocarpa''. * Medigenin *Sclareol * Stemodene See also * Abietane Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes. Abietanes are found in the ... References {{reflist Diterpenes Decalins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol * Geranyl pyrophosphate Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |